Inhibition of p53-dependent transcription by BOX-I phospho-peptide mimetics that bind to p300
- PMID: 11258706
- PMCID: PMC1083821
- DOI: 10.1093/embo-reports/kve025
Inhibition of p53-dependent transcription by BOX-I phospho-peptide mimetics that bind to p300
Abstract
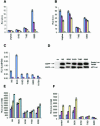
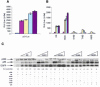
The N-terminal BOX-I domain of p53 containing a docking site for the negative regulator MDM2 and the positive effector p300, harbours two recently identified phosphorylation sites at Thr18 or Ser20O whose affect on p300 is undefined. Biochemical assays demonstrate that although MDM2 binding is inhibited by these phosphorylations, p300 binding is strikingly stabilized by Thr18 or Ser20 phosphorylation. Introducing EGFP-BOX-I domain peptides with an aspartate substitution at Thr18 or Ser20 induced a significant inhibition of endogenous p53-dependent transcription in cycling cells, in irradiated cells, as well as in cells transiently co-transfected with p300 and p53. In contrast an EGFP-wild-type BOX-I domain peptide stimulated p53 activity via inhibition of MDM2 protein binding. These results suggest that phosphorylation of p53 at Thr18 or Ser20 can activate p53 by stabilizing the p300-p53 complex and also identify a class of small molecular weight ligands capable of selective discrimination between MDM2- and p300-dependent activities.
Figures
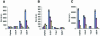
References
-
- Bottger A., Bottger, V., Sparks, A., Liu, W.L., Howard, S.F. and Lane, D.P. (1997) Design of a synthetic Mdm2-binding mini protein that activates the p53 response in vivo. Curr. Biol., 7, 860–869. - PubMed
-
- Burch L.R., Midgley, C.A., Currie, R.A., Lane, D.P. and Hupp, T.R. (2000) Mdm2 binding to a conformationally sensitive domain on p53 can be modulated by RNA. FEBS Lett., 472, 93–98. - PubMed
-
- Craig A.L., Blaydes, J.P., Burch, L.R., Thompson, A.M. and Hupp, T.R. (1999a) Dephosphorylation of p53 at Ser20 after cellular exposure to low levels of non-ionizing radiation. Oncogene, 18, 6305–6312. - PubMed
-
- Craig A.L., Burch, L., Vojtesek, B., Mikutowska, J., Thompson, A. and Hupp, T.R. (1999b) Novel phosphorylation sites of human tumour suppressor protein p53 at Ser20 and Thr18 that disrupt the binding of mdm2 (mouse double minute 2) protein are modified in human cancers. Biochem. J., 342, 133–141. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

