A role for chemokine receptor transactivation in growth factor signaling
- PMID: 11258708
- PMCID: PMC1083823
- DOI: 10.1093/embo-reports/kve027
A role for chemokine receptor transactivation in growth factor signaling
Abstract
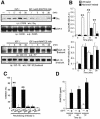
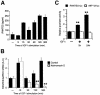
Complex cell responses require the integration of signals delivered through different pathways. We show that insulin-like growth factor (IGF)-I induces specific transactivation of the Gi-coupled chemokine receptor CCR5, triggering its tyrosine phosphorylation and Galpha recruitment. This transactivation occurs via a mechanism involving transcriptional upregulation and secretion of RANTES, the natural CCR5 ligand. CCR5 transactivation is an essential downstream signal in IGF-I-induced cell chemotaxis, as abrogation of CCR5 function with a transdominant-negative KDELccr5A32 mutant abolishes IGF-I-induced migration. The relevance of this transactivation pathway was shown in vivo, as KDELccr5A32 overexpression prevents invasion by highly metastatic tumor cells; conversely, RANTES overexpression confers built-in invasive capacity on a non-invasive tumor cell line. Our results suggest that this extracellular growth factor-chemokine network represents a general mechanism connecting tumorigenesis and inflammation.
Figures



Similar articles
-
Transactivation of CXCR4 by the insulin-like growth factor-1 receptor (IGF-1R) in human MDA-MB-231 breast cancer epithelial cells.J Biol Chem. 2005 Dec 2;280(48):39701-8. doi: 10.1074/jbc.M509829200. Epub 2005 Sep 19. J Biol Chem. 2005. PMID: 16172123
-
G protein subtype-specific signaling bias in a series of CCR5 chemokine analogs.Sci Signal. 2018 Oct 16;11(552):eaao6152. doi: 10.1126/scisignal.aao6152. Sci Signal. 2018. PMID: 30327411
-
Molecular anatomy of CCR5 engagement by physiologic and viral chemokines and HIV-1 envelope glycoproteins: differences in primary structural requirements for RANTES, MIP-1 alpha, and vMIP-II Binding.J Mol Biol. 2001 Nov 9;313(5):1181-93. doi: 10.1006/jmbi.2001.5086. J Mol Biol. 2001. PMID: 11700073
-
The potential to target CCL5/CCR5 in breast cancer.Expert Opin Ther Targets. 2014 Nov;18(11):1265-75. doi: 10.1517/14728222.2014.949238. Epub 2014 Sep 26. Expert Opin Ther Targets. 2014. PMID: 25256399 Review.
-
Inhibition of the CCL5/CCR5 Axis against the Progression of Gastric Cancer.Int J Mol Sci. 2018 May 16;19(5):1477. doi: 10.3390/ijms19051477. Int J Mol Sci. 2018. PMID: 29772686 Free PMC article. Review.
Cited by
-
Alpha-fetoprotein contributes to THP-1 cell invasion and chemotaxis via protein kinase and Gi-protein-dependent pathways.Mol Cell Biochem. 2013 Jul;379(1-2):283-93. doi: 10.1007/s11010-013-1650-6. Epub 2013 Apr 25. Mol Cell Biochem. 2013. Retraction in: Mol Cell Biochem. 2023 Nov;478(11):2607. doi: 10.1007/s11010-023-04675-6. PMID: 23615710 Retracted.
-
Differential effect of growth factors on invasion and proliferation of endocrine resistant breast cancer cells.PLoS One. 2012;7(7):e41847. doi: 10.1371/journal.pone.0041847. Epub 2012 Jul 30. PLoS One. 2012. PMID: 22860018 Free PMC article.
-
Synaptic plasticity via receptor tyrosine kinase/G-protein-coupled receptor crosstalk.Cell Rep. 2024 Jan 23;43(1):113595. doi: 10.1016/j.celrep.2023.113595. Epub 2023 Dec 19. Cell Rep. 2024. PMID: 38117654 Free PMC article.
-
Host microenvironment in breast cancer development: inflammatory cells, cytokines and chemokines in breast cancer progression: reciprocal tumor-microenvironment interactions.Breast Cancer Res. 2003;5(1):31-6. doi: 10.1186/bcr554. Epub 2002 Oct 28. Breast Cancer Res. 2003. PMID: 12559043 Free PMC article. Review.
-
SOD3 improves the tumor response to chemotherapy by stabilizing endothelial HIF-2α.Nat Commun. 2018 Feb 8;9(1):575. doi: 10.1038/s41467-018-03079-1. Nat Commun. 2018. PMID: 29422508 Free PMC article.
References
-
- Benkirane M., Jin, D., Chun, R., Koup, R. and Jeang, K. (1997) Mechanism of transdominant inhibition of CCR5-mediated HIV-1 infection by ccr5Δ32. J. Biol. Chem., 272, 30603–30606. - PubMed
-
- Hallak H., Seiler, A., Green, J., Ross, B. and Rubin, R. (2000) Association of heterotrimeric Gi with the insulin-like growth factor-I receptor. Release of Gβγ subunits upon receptor activation. J. Biol. Chem., 275, 2255–2258. - PubMed
-
- Kanzaki M., Nie, L., Shibata, H. and Kojima, I. (1997) Activation of a calcium-permeable cation channel CD20 expressed in Balb/c 3T3 cells by insulin-like growth factor-I. J. Biol. Chem., 272, 4964–4969. - PubMed
-
- Kim J., Yu, W., Kovalski, K. and Ossowski, L. (1998) Requirement for specific proteases in cancer cell intravasation as revealed by a novel semiquantitative PCR-based assay. Cell, 94, 353–362. - PubMed
-
- Liu R. et al. (1996) Homozygous defect in HIV-1 coreceptor accounts for resistence of some multiply-exposed individuals to HIV-1 infection. Cell, 86, 367–377. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

