Association of insulin receptor substrate 1 (IRS-1) y895 with Grb-2 mediates the insulin signaling involved in IRS-1-deficient brown adipocyte mitogenesis
- PMID: 11259577
- PMCID: PMC86861
- DOI: 10.1128/MCB.21.7.2269-2280.2001
Association of insulin receptor substrate 1 (IRS-1) y895 with Grb-2 mediates the insulin signaling involved in IRS-1-deficient brown adipocyte mitogenesis
Abstract
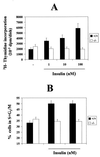
We have recently generated immortalized fetal brown adipocyte cell lines from insulin receptor substrate 1 (IRS-1) knockout mice and demonstrated an impairment in insulin-induced lipid synthesis as compared to wild-type cell lines. In this study, we investigated the consequences of IRS-1 deficiency on mitogenesis in response to insulin. The lack of IRS-1 resulted in the inability of insulin-stimulated IRS-1-deficient brown adipocytes to increase DNA synthesis and enter into S/G2/M phases of the cell cycle. These cells showed a severe impairment in activating mitogen-activated protein kinase kinase (MEK1/2) and p42-p44 mitogen-activated protein kinase (MAPK) upon insulin stimulation. IRS-1-deficient cells also lacked tyrosine phosphorylation of SHC and showed no SHC-Grb-2 association in response to insulin. The mitogenic response to insulin could be partially restored by enhancing IRS-2 tyrosine phosphorylation and its association with Grb-2 by inhibition of phosphatidylinositol 3-kinase activity through a feedback mechanism. Reconstitution of IRS-1-deficient brown adipocytes with wild-type IRS-1 restored insulin-induced IRS-1 and SHC tyrosine phosphorylation and IRS-1-Grb-2, IRS-1-SHC, and SHC-Grb-2 associations, leading to the activation of MAPK and enhancement of DNA synthesis. Reconstitution of IRS-1-deficient brown adipocytes with the IRS-1 mutant Tyr895Phe, which lacks IRS-1-Grb-2 binding, restored SHC-IRS-1 association and SHC-Grb-2 association. However, the lack of IRS-1-Grb-2 association impaired MAPK activation and DNA synthesis in insulin-stimulated mutant cells. These data provide strong evidence for an essential role of IRS-1 and its direct association with Grb-2 in the insulin signaling pathway leading to MAPK activation and mitogenesis in brown adipocytes.
Figures








References
-
- Araki E, Lipes M A, Patti M E, Brüning J C, Haag III B L, Johnson R S, Kahn C R. Alternative pathway of insulin signalling in mice made with targeted disruption of the IRS-1 gene. Nature. 1994;372:186–190. - PubMed
-
- Baserga R. Growth regulation of the PCNA gene. J Cell Sci. 1991;98:433–436. - PubMed
-
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
