p38 mitogen-activated protein kinase-dependent activation of protein phosphatases 1 and 2A inhibits MEK1 and MEK2 activity and collagenase 1 (MMP-1) gene expression
- PMID: 11259586
- PMCID: PMC86870
- DOI: 10.1128/MCB.21.7.2373-2383.2001
p38 mitogen-activated protein kinase-dependent activation of protein phosphatases 1 and 2A inhibits MEK1 and MEK2 activity and collagenase 1 (MMP-1) gene expression
Abstract
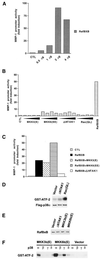
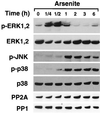
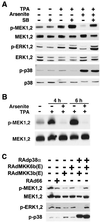
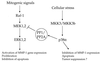
Degradation of collagenous extracellular matrix by collagenase 1 (also known as matrix metalloproteinase 1 [MMP-1]) plays a role in the pathogenesis of various destructive disorders, such as rheumatoid arthritis, chronic ulcers, and tumor invasion and metastasis. Here, we have investigated the role of distinct mitogen-activated protein kinase (MAPK) pathways in the regulation of MMP-1 gene expression. The activation of the extracellular signal-regulated kinase 1 (ERK1)/ERK2 (designated ERK1,2) pathway by oncogenic Ras, constitutively active Raf-1, or phorbol ester resulted in potent stimulation of MMP-1 promoter activity and mRNA expression. In contrast, activation of stress-activated c-Jun N-terminal kinase and p38 pathways by expression of constitutively active mutants of Rac, transforming growth factor beta-activated kinase 1 (TAK1), MAPK kinase 3 (MKK3), or MKK6 or by treatment with arsenite or anisomycin did not alone markedly enhance MMP-1 promoter activity. Constitutively active MKK6 augmented Raf-1-mediated activation of the MMP-1 promoter, whereas active mutants of TAK1 and MKK3b potently inhibited the stimulatory effect of Raf-1. Activation of p38 MAPK by arsenite also potently abrogated stimulation of MMP-1 gene expression by constitutively active Ras and Raf-1 and by phorbol ester. Specific activation of p38alpha by adenovirus-delivered constitutively active MKK3b resulted in potent inhibition of the activity of ERK1,2 and its upstream activator MEK1,2. Furthermore, arsenite prevented phorbol ester-induced phosphorylation of ERK1,2 kinase-MEK1,2, and this effect was dependent on p38-mediated activation of protein phosphatase 1 (PP1) and PP2A. These results provide evidence that activation of signaling cascade MKK3-MKK3b-->p38alpha blocks the ERK1,2 pathway at the level of MEK1,2 via PP1-PP2A and inhibits the activation of MMP-1 gene expression.
Figures







Similar articles
-
Enhancement of fibroblast collagenase-1 (MMP-1) gene expression by tumor promoter okadaic acid is mediated by stress-activated protein kinases Jun N-terminal kinase and p38.Matrix Biol. 1998 Dec;17(8-9):547-57. doi: 10.1016/s0945-053x(98)90107-x. Matrix Biol. 1998. PMID: 9923649
-
Transforming growth factor-beta induces collagenase-3 expression by human gingival fibroblasts via p38 mitogen-activated protein kinase.J Biol Chem. 1999 Dec 24;274(52):37292-300. doi: 10.1074/jbc.274.52.37292. J Biol Chem. 1999. PMID: 10601295
-
Fibroblast growth factor receptor signaling activates the human interstitial collagenase promoter via the bipartite Ets-AP1 element.Mol Endocrinol. 1997 Jul;11(8):1129-44. doi: 10.1210/mend.11.8.9958. Mol Endocrinol. 1997. PMID: 9212060
-
Pharmacological inhibitors of MAPK pathways.Trends Pharmacol Sci. 2002 Jan;23(1):40-5. doi: 10.1016/s0165-6147(00)01865-4. Trends Pharmacol Sci. 2002. PMID: 11804650 Review.
-
Mitogen-activated protein kinase cascade and transcription factors: the opposite role of MKK3/6-p38K and MKK1-MAPK.Nephrol Dial Transplant. 1999;14 Suppl 1:45-7. doi: 10.1093/ndt/14.suppl_1.45. Nephrol Dial Transplant. 1999. PMID: 10048449 Review.
Cited by
-
Roles of the Ras-MEK-mitogen-activated protein kinase and phosphatidylinositol 3-kinase-Akt-mTOR pathways in Jaagsiekte sheep retrovirus-induced transformation of rodent fibroblast and epithelial cell lines.J Virol. 2005 Apr;79(7):4440-50. doi: 10.1128/JVI.79.7.4440-4450.2005. J Virol. 2005. PMID: 15767444 Free PMC article.
-
Differential pharmacological behaviour of p38 inhibitors in regulating the LPS-induced TNF-α production in human and rat whole blood in vitro.Inflammation. 2011 Apr;34(2):119-32. doi: 10.1007/s10753-010-9215-2. Inflammation. 2011. PMID: 20446028
-
PAK1 regulates breast cancer cell invasion through secretion of matrix metalloproteinases in response to prolactin and three-dimensional collagen IV.Mol Endocrinol. 2013 Jul;27(7):1048-64. doi: 10.1210/me.2012-1322. Epub 2013 Jun 6. Mol Endocrinol. 2013. PMID: 23744893 Free PMC article.
-
Anthocyanins protected hearts against ischemic injury by reducing MMP-2 activity via Akt/P38 pathways.Am J Transl Res. 2016 Feb 15;8(2):1100-7. eCollection 2016. Am J Transl Res. 2016. PMID: 27158396 Free PMC article.
-
Computational model predicts paracrine and intracellular drivers of fibroblast phenotype after myocardial infarction.Matrix Biol. 2020 Sep;91-92:136-151. doi: 10.1016/j.matbio.2020.03.007. Epub 2020 Mar 21. Matrix Biol. 2020. PMID: 32209358 Free PMC article. Review.
References
-
- Abraham D, Podar K, Pacher M, Kubicek M, Welzel N, Hemmings B A, Dilworth S M, Mischak H, Kolch W, Baccarini M. Raf-1-associated PP2A as a positive regulator of kinase activation. J Biol Chem. 2000;275:22300–22304. - PubMed
-
- Bruder J T, Heidecker G, Rapp U R. Serum, TPA-, and Ras-induced expression from Ap-1/Ets driven promoters requires Raf-1 kinase. Genes Dev. 1992;6:545–556. - PubMed
-
- Chen Y, Lin-Shiau S, Lin J. Involvement of reactive oxygen species and caspase 3 activation in arsenite-induced apoptosis. J Cell Physiol. 1998;177:324–333. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
