Essential role of insulin receptor substrate 1 (IRS-1) and IRS-2 in adipocyte differentiation
- PMID: 11259600
- PMCID: PMC86884
- DOI: 10.1128/MCB.21.7.2521-2532.2001
Essential role of insulin receptor substrate 1 (IRS-1) and IRS-2 in adipocyte differentiation
Abstract
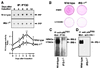
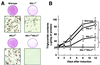
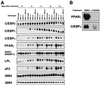
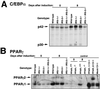
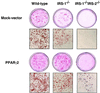
To investigate the role of insulin receptor substrate 1 (IRS-1) and IRS-2, the two ubiquitously expressed IRS proteins, in adipocyte differentiation, we established embryonic fibroblast cells with four different genotypes, i.e., wild-type, IRS-1 deficient (IRS-1(-/-)), IRS-2 deficient (IRS-2(-/-)), and IRS-1 IRS-2 double deficient (IRS-1(-/-) IRS-2(-/-)), from mouse embryos of the corresponding genotypes. The abilities of IRS-1(-/-) cells and IRS-2(-/-) cells to differentiate into adipocytes are approximately 60 and 15%, respectively, lower than that of wild-type cells, at day 8 after induction and, surprisingly, IRS-1(-/-) IRS-2(-/-) cells have no ability to differentiate into adipocytes. The expression of CCAAT/enhancer binding protein alpha (C/EBPalpha) and peroxisome proliferator-activated receptor gamma (PPARgamma) is severely decreased in IRS-1(-/-) IRS-2(-/-) cells at both the mRNA and the protein level, and the mRNAs of lipoprotein lipase and adipocyte fatty acid binding protein are severely decreased in IRS-1(-/-) IRS-2(-/-) cells. Phosphatidylinositol 3-kinase (PI 3-kinase) activity that increases during adipocyte differentiation is almost completely abolished in IRS-1(-/-) IRS-2(-/-) cells. Treatment of wild-type cells with a PI 3-kinase inhibitor, LY294002, markedly decreases the expression of C/EBPalpha and PPARgamma, a result which is associated with a complete block of adipocyte differentiation. Moreover, histologic analysis of IRS-1(-/-) IRS-2(-/-) double-knockout mice 8 h after birth reveals severe reduction in white adipose tissue mass. Our results suggest that IRS-1 and IRS-2 play a crucial role in the upregulation of the C/EBPalpha and PPARgamma expression and adipocyte differentiation.
Figures




References
-
- Cao Z, Umek R M, McKnight S L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3–L1 cells. Genes Dev. 1991;5:1538–1552. - PubMed
-
- Catalioto R M, Gailard D, Ailhaud G, Negrel R. Terminal differentiation of mouse preadipocyte cells: the mitogenic-adipogenic role of growth hormone is mediated by the protein kinase C signalling pathway. Growth Factors. 1992;6:255–264. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
