2-arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor
- PMID: 11259648
- PMCID: PMC31108
- DOI: 10.1073/pnas.061029898
2-arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor
Abstract
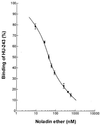
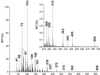
Two types of endogenous cannabinoid-receptor agonists have been identified thus far. They are the ethanolamides of polyunsaturated fatty acids--arachidonoyl ethanolamide (anandamide) is the best known compound in the amide series--and 2-arachidonoyl glycerol, the only known endocannabinoid in the ester series. We report now an example of a third, ether-type endocannabinoid, 2-arachidonyl glyceryl ether (noladin ether), isolated from porcine brain. The structure of noladin ether was determined by mass spectrometry and nuclear magnetic resonance spectroscopy and was confirmed by comparison with a synthetic sample. It binds to the CB(1) cannabinoid receptor (K(i) = 21.2 +/- 0.5 nM) and causes sedation, hypothermia, intestinal immobility, and mild antinociception in mice. It binds weakly to the CB(2) receptor (K(i) > 3 microM).
Figures
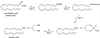


References
-
- Devane W A, Hanuš L, Breuer A, Pertwee R G, Stevenson L A, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Science. 1992;258:1946–1949. - PubMed
-
- Hanuš L, Gopher A, Almog S, Mechoulam R. J Med Chem. 1993;36:3032–3034. - PubMed
-
- Mechoulam R, Ben-Shabat S, Hanuš L, Ligumsky M, Kaminski N E, Schatz A R, Gopher A, Almog S, Martin B R, Compton D R, et al. Biochem Pharmacol. 1995;50:83–90. - PubMed
-
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. Biochem Biophys Res Commun. 1995;215:89–97. - PubMed
-
- Mechoulam R, Fride E, Di Marzo V. Eur J Pharmacol. 1998;359:1–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

