Nicotinic cholinergic signaling in hippocampal astrocytes involves calcium-induced calcium release from intracellular stores
- PMID: 11259680
- PMCID: PMC31194
- DOI: 10.1073/pnas.071540198
Nicotinic cholinergic signaling in hippocampal astrocytes involves calcium-induced calcium release from intracellular stores
Abstract
In this report we provide evidence that neuronal nicotinic acetylcholine receptors (nAChRs) are present on hippocampal astrocytes and their activation produces rapid currents and calcium transients. Our data indicate that these responses obtained from astrocytes are primarily mediated by an AChR subtype that is functionally blocked by alpha-bungarotoxin (alpha Bgt) and contains the alpha7 subunit (alpha Bgt-AChRs). Furthermore, their action is unusual in that they effectively increase intracellular free calcium concentrations by activating calcium-induced calcium release from intracellular stores, triggered by influx through the receptor channels. These results reveal a mechanism by which alpha Bgt-AChRs on astrocytes can efficiently modulate calcium signaling in the central nervous system in a manner distinct from that observed with these receptors on neurons.
Figures

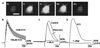
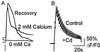
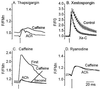
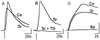
Comment in
-
New functions for glia in the brain.Proc Natl Acad Sci U S A. 2001 Mar 27;98(7):3631-2. doi: 10.1073/pnas.081073198. Proc Natl Acad Sci U S A. 2001. PMID: 11274377 Free PMC article. No abstract available.
Similar articles
-
Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. IV. Regulation by external Ca++ of alpha-bungarotoxin-sensitive receptor function and of rectification induced by internal Mg++.J Pharmacol Exp Ther. 1996 Apr;277(1):432-44. J Pharmacol Exp Ther. 1996. PMID: 8613952
-
Novel subpopulation of neuronal acetylcholine receptors among those binding alpha-bungarotoxin.Mol Pharmacol. 1995 Apr;47(4):717-25. Mol Pharmacol. 1995. PMID: 7723732
-
Modulation of alpha7 nicotinic receptor-mediated calcium influx by nicotinic agonists.Mol Pharmacol. 1997 Mar;51(3):499-506. Mol Pharmacol. 1997. PMID: 9058606
-
Differential effects of chronic drug treatment on alpha3* and alpha7 nicotinic receptor binding sites, in hippocampal neurones and SH-SY5Y cells.Br J Pharmacol. 2001 Aug;133(8):1286-95. doi: 10.1038/sj.bjp.0704207. Br J Pharmacol. 2001. PMID: 11498514 Free PMC article.
-
Elevation of intracellular calcium levels in neurons by nicotinic acetylcholine receptors.Mol Neurobiol. 1996 Apr;12(2):117-31. doi: 10.1007/BF02740649. Mol Neurobiol. 1996. PMID: 8818146 Review.
Cited by
-
Nicotine and behavioral sensitization.J Mol Neurosci. 2010 Jan;40(1-2):154-63. doi: 10.1007/s12031-009-9230-7. Epub 2009 Aug 11. J Mol Neurosci. 2010. PMID: 19669944 Free PMC article. Review.
-
A dominant role for the beta 4 nicotinic receptor subunit in nicotinic modulation of glomerular microcircuits in the mouse olfactory bulb.J Neurophysiol. 2018 Oct 1;120(4):2036-2048. doi: 10.1152/jn.00925.2017. Epub 2018 Aug 8. J Neurophysiol. 2018. PMID: 30089021 Free PMC article.
-
Neuroprotection by nicotine in mouse primary cortical cultures involves activation of calcineurin and L-type calcium channel inactivation.J Neurosci. 2003 Nov 5;23(31):10093-9. doi: 10.1523/JNEUROSCI.23-31-10093.2003. J Neurosci. 2003. PMID: 14602824 Free PMC article.
-
Modulation of multiple memory systems: from neurotransmitters to metabolic substrates.Hippocampus. 2013 Nov;23(11):1053-65. doi: 10.1002/hipo.22182. Hippocampus. 2013. PMID: 23929581 Free PMC article. Review.
-
Regulation of Inflammation by IRAK-M Pathway Can Be Associated with nAchRalpha7 Activation and COVID-19.Mol Neurobiol. 2024 Feb;61(2):581-592. doi: 10.1007/s12035-023-03567-6. Epub 2023 Aug 29. Mol Neurobiol. 2024. PMID: 37640915 Review.
References
-
- Role L W, Berg D K. Neuron. 1996;16:1077–1085. - PubMed
-
- MacDermott A B, Role L W, Siegelbaum S A. Annu Rev Neurosci. 1999;22:443–485. - PubMed
-
- Mansvelder H D, McGehee D S. Neuron. 2000;27:349–357. - PubMed
-
- Leonard S, Breese C, Adams C, Benhammou K, Gault J, Stevens K, Lee M, Adler L, Olincy A, Ross R, et al. Eur J Pharmacol. 2000;393:237–242. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

