Role of the cytoplasmic tyrosines of beta 1A integrins in transformation by v-src
- PMID: 11259684
- PMCID: PMC31134
- DOI: 10.1073/pnas.240456398
Role of the cytoplasmic tyrosines of beta 1A integrins in transformation by v-src
Abstract
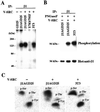
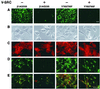
GD25 cells lacking beta 1 integrins or expressing beta 1A with mutations of conserved cytoplasmic tyrosines (Y783, Y795) to phenylalanine have poor directed migration to platelet-derived growth factor or lysophosphatidic acid when compared with GD25 cells expressing wild-type beta 1A. We studied the effects of v-src on these cells. Transformation with v-src caused tyrosine and serine phosphorylation of wild-type beta1 A but not of Y783/795F doubly mutated beta 1A. v-src-transformed cells had rounded and/or fusiform morphology and poor assembly of fibronectin matrix. Adhesion to fibronectin or laminin and coupling of focal contacts to actin-containing cytoskeleton were preserved in transformed Y783/795F cells but lost on transformation when beta 1A was wild type. Transformed Y783/795F cells also retained ability, albeit limited, to migrate across filters, whereas transformed cells with wild-type beta 1A were unable to transverse filters. Studies of single tyrosine mutants showed that the more important tyrosine for retaining ability to adhere, assemble focal contacts, and migrate is Y783. These results suggest that overactive phosphorylation of cytoplasmic residues of beta 1A, particularly Y783, accounts in part for the phenotype of v-src-transformed cells.
Figures
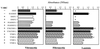


References
-
- Jove R, Hanafusa H. Annu Rev Cell Biol. 1987;3:31–56. - PubMed
-
- Parsons J T, Weber M J. Curr Top Microbiol Immunol. 1989;147:79–127. - PubMed
-
- Burridge K, Fath K, Kelly T, Nuckolls G, Turner C E. Annu Rev Cell Biol. 1988;4:487–525. - PubMed
-
- Chambers A F, Tuck A B. Anticancer Res. 1988;8:861–872. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

