Involvement of multiple human cytochromes P450 in the liver microsomal metabolism of astemizole and a comparison with terfenadine
- PMID: 11259984
- PMCID: PMC2014443
- DOI: 10.1111/j.1365-2125.2001.01292.x
Involvement of multiple human cytochromes P450 in the liver microsomal metabolism of astemizole and a comparison with terfenadine
Abstract
Aims: The aims of the present study were to investigate the metabolism of astemizole in human liver microsomes, to assess possible pharmacokinetic drug-interactions with astemizole and to compare its metabolism with terfenadine, a typical H1 receptor antagonist known to be metabolized predominantly by CYP3A4.
Methods: Astemizole or terfenadine were incubated with human liver microsomes or recombinant cytochromes P450 in the absence or presence of chemical inhibitors and antibodies.
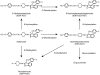
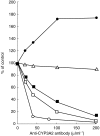
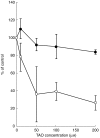
Results: Troleandomycin, a CYP3A4 inhibitor, markedly reduced the oxidation of terfenadine (26% of controls) in human liver microsomes, but showed only a marginal inhibition on the oxidation of astemizole (81% of controls). Three metabolites of astemizole were detected in a liver microsomal system, i.e. desmethylastemizole (DES-AST), 6-hydroxyastemizole (6OH-AST) and norastemizole (NOR-AST) at the ratio of 7.4 : 2.8 : 1. Experiments with recombinant P450s and antibodies indicate a negligible role for CYP3A4 on the main metabolic route of astemizole, i.e. formation of DES-AST, although CYP3A4 may mediate the relatively minor metabolic routes to 6OH-AST and NOR-AST. Recombinant CYP2D6 catalysed the formation of 6OH-AST and DES-AST. Studies with human liver microsomes, however, suggest a major role for a mono P450 in DES-AST formation.
Conclusions: In contrast to terfenadine, a minor role for CYP3A4 and involvement of multiple P450 isozymes are suggested in the metabolism of astemizole. These differences in P450 isozymes involved in the metabolism of astemizole and terfenadine may associate with distinct pharmacokinetic influences observed with coadministration of drugs metabolized by CYP3A4.
Figures
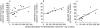
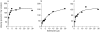
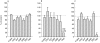
Similar articles
-
Identifying a selective substrate and inhibitor pair for the evaluation of CYP2J2 activity.Drug Metab Dispos. 2012 May;40(5):943-51. doi: 10.1124/dmd.111.043505. Epub 2012 Feb 10. Drug Metab Dispos. 2012. PMID: 22328583 Free PMC article.
-
Identification of human liver cytochrome P450 enzymes that metabolize the nonsedating antihistamine loratadine. Formation of descarboethoxyloratadine by CYP3A4 and CYP2D6.Biochem Pharmacol. 1996 Jan 26;51(2):165-72. doi: 10.1016/0006-2952(95)02169-8. Biochem Pharmacol. 1996. PMID: 8615885
-
In vitro metabolism of terfenadine by a purified recombinant fusion protein containing cytochrome P4503A4 and NADPH-P450 reductase. Comparison to human liver microsomes and precision-cut liver tissue slices.Drug Metab Dispos. 1995 Jul;23(7):765-75. Drug Metab Dispos. 1995. PMID: 7587966
-
Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition.Clin Pharmacokinet. 2000 Jan;38(1):41-57. doi: 10.2165/00003088-200038010-00003. Clin Pharmacokinet. 2000. PMID: 10668858 Review.
-
Protein subinteractomes of human microsomal cytochromes P450.Mol Biol Rep. 2025 Feb 12;52(1):226. doi: 10.1007/s11033-025-10341-5. Mol Biol Rep. 2025. PMID: 39937310 Review.
Cited by
-
Investigating the contribution of CYP2J2 to ritonavir metabolism in vitro and in vivo.Biochem Pharmacol. 2014 Sep 1;91(1):109-18. doi: 10.1016/j.bcp.2014.06.020. Epub 2014 Jun 25. Biochem Pharmacol. 2014. PMID: 24973543 Free PMC article.
-
Random walk with restart on multilayer networks: from node prioritisation to supervised link prediction and beyond.BMC Bioinformatics. 2024 Feb 14;25(1):70. doi: 10.1186/s12859-024-05683-z. BMC Bioinformatics. 2024. PMID: 38355439 Free PMC article.
-
Identifying a selective substrate and inhibitor pair for the evaluation of CYP2J2 activity.Drug Metab Dispos. 2012 May;40(5):943-51. doi: 10.1124/dmd.111.043505. Epub 2012 Feb 10. Drug Metab Dispos. 2012. PMID: 22328583 Free PMC article.
-
Optimization of the bacterial cytochrome P450 BM3 system for the production of human drug metabolites.Int J Mol Sci. 2012 Nov 28;13(12):15901-24. doi: 10.3390/ijms131215901. Int J Mol Sci. 2012. PMID: 23443101 Free PMC article. Review.
-
CYP2J2 Expression in Adult Ventricular Myocytes Protects Against Reactive Oxygen Species Toxicity.Drug Metab Dispos. 2018 Apr;46(4):380-386. doi: 10.1124/dmd.117.078840. Epub 2018 Jan 17. Drug Metab Dispos. 2018. PMID: 29343610 Free PMC article.
References
-
- Richards DM, Brogden RN, Heel RC, Speight TM, Avery GS. Astemizole, a review of its pharmacodynamic properties and therapeutic efficacy. Drugs. 1984;28:38–61. - PubMed
-
- Meuldermans W, Hendrickx J, Lauwers W, Hurkmans R, Swysen E, Heykants J. Excretion and biotransformation of astemizole in rats, guinea-pigs, dogs, and man. Drug Dev Res. 1986;8:37–51.
-
- Heykants J, Peer AV, Woestenborghs R, Jageneau A, Bussche GV. Dose-proportionality, bioavailability, and steady-state kinetics of astemizole in man. Drug Dev Res. 1986;8:71–78.
-
- Clark A, Love H. Astemizole–induced ventricular arrhythmias: an unexpected cause of convulsion. Int J Cardiol. 1991;33:165–167. - PubMed
-
- Nightingale SL. Warning issued on non-sedating antihistamines terfenadine and astemizole. JAMA. 1992;268:705. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

