Influence of gender and oral contraceptives on CYP2D6 and CYP2C19 activity in healthy volunteers
- PMID: 11259990
- PMCID: PMC2014435
- DOI: 10.1111/j.1365-2125.2001.01328.x
Influence of gender and oral contraceptives on CYP2D6 and CYP2C19 activity in healthy volunteers
Abstract
Aims: The study was carried out in order to assess the effects of gender and the use of oral contraceptives (OCs) on CYP2D6 and CYP2C19 activities in healthy volunteers.
Methods: Six hundred and eleven Caucasian volunteers (330 males and 281 females; age range 18-49 years) were phenotyped with respect to CYP2D6 and CYP2C19 by means of the probe drugs dextromethorphan and mephenytoin, respectively. Extensive metabolisers were selected for this study.
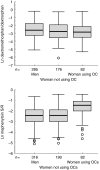
Results: The median dextromethorphan/dextrorphan metabolic ratio in non-OC using females was significantly lower than in males (0.067 vs 0.080; P = 0.033) (mean difference in ln dextromethorphan/dextrorphan metabolic ratio 0.023, 95% CI 0.03-0.43). For the mephenytoin S/R ratio, no such difference was observed. However, OC using females had a significantly higher median mephenytoin S/R ratio than non-OC using females (0.230 vs 0.090; P < 0.001) (mean difference in ln mephenytoin S/R ratio 0.082, 95% CI 0.60-1.04). Moreover, females using combined OCs had a significantly higher median ratio than females using OCs with progestins only (median 0.258 vs 0.135; P = 0.008) (mean difference in ln mephenytoin S/R ratio 0.82, 95% CI 0.21-1.34).
Conclusions: Given certain assumptions, the study indicates that females in the fertile age have a slightly higher CYP2D6 activity compared with males. There was no evidence of a gender difference in CYP2C19 activity. The use of combined OCs reduces the activity of CYP2C19, an effect that seems to be related to the ethinyloestradiol component.
Figures
Similar articles
-
CYP2D6 and CYP2C19 activity in a large population of Dutch healthy volunteers: indications for oral contraceptive-related gender differences.Eur J Clin Pharmacol. 1999 May;55(3):177-84. doi: 10.1007/s002280050615. Eur J Clin Pharmacol. 1999. PMID: 10379632
-
Genetic polymorphism of CYP2D6 and CYP2C19 metabolism determined by phenotyping Israeli ethnic groups.Ther Drug Monit. 2000 Oct;22(5):510-6. doi: 10.1097/00007691-200010000-00002. Ther Drug Monit. 2000. PMID: 11034254
-
Assessment of CYP2D6 and CYP2C19 activity in vivo in humans: a cocktail study with dextromethorphan and chloroguanide alone and in combination.Clin Pharmacol Ther. 1999 Dec;66(6):582-8. doi: 10.1053/cp.1999.v66.103401001. Clin Pharmacol Ther. 1999. PMID: 10613613 Clinical Trial.
-
Investigation of xenobiotic metabolism by CYP2D6 and CYP2C19: importance of enantioselective analytical methods.J Chromatogr B Biomed Appl. 1996 Mar 29;678(1):73-92. doi: 10.1016/0378-4347(95)00229-4. J Chromatogr B Biomed Appl. 1996. PMID: 8861658 Review.
-
The procarcinogen hypothesis for bladder cancer: activities of individual drug metabolizing enzymes as risk factors.Pharmacogenetics. 1995;5 Spec No:S97-102. doi: 10.1097/00008571-199512001-00009. Pharmacogenetics. 1995. PMID: 7581498 Review.
Cited by
-
Personalizing atomoxetine dosing in children with ADHD: what can we learn from current supporting evidence.Eur J Clin Pharmacol. 2023 Mar;79(3):349-370. doi: 10.1007/s00228-022-03449-1. Epub 2023 Jan 16. Eur J Clin Pharmacol. 2023. PMID: 36645468 Review.
-
Pharmacokinetics of omeprazole and its metabolites in three phases of menstrual cycle.Eur J Drug Metab Pharmacokinet. 2015 Mar;40(1):13-22. doi: 10.1007/s13318-013-0167-4. Epub 2014 Jan 5. Eur J Drug Metab Pharmacokinet. 2015. PMID: 24390778 Clinical Trial.
-
Sex and gender differences in polypharmacy in persons with dementia: A scoping review.SAGE Open Med. 2019 Apr 22;7:2050312119845715. doi: 10.1177/2050312119845715. eCollection 2019. SAGE Open Med. 2019. PMID: 31041100 Free PMC article.
-
Effect of isotretinoin on CYP2D6 and CYP3A activity in patients with severe acne.Br J Clin Pharmacol. 2024 Mar;90(3):759-768. doi: 10.1111/bcp.15938. Epub 2023 Nov 21. Br J Clin Pharmacol. 2024. PMID: 37864393 Free PMC article.
-
Sex and Gender Differences in Clinical Pharmacology: Implications for Transgender Medicine.Clin Pharmacol Ther. 2021 Oct;110(4):897-908. doi: 10.1002/cpt.2234. Epub 2021 May 2. Clin Pharmacol Ther. 2021. PMID: 33763856 Free PMC article. Review.
References
-
- Bertilsson L. Geographical/interracial differences in polymorphic drug oxidation. Clin Pharmacokin. 1995;29:169–173. - PubMed
-
- Harris RZ, Benet LZ, Schwartz JB. Gender effects in pharmacokinetics and pharmacodynamics. Drugs. 1995;50:222–239. - PubMed
-
- Llerena A, Cobaleda J, Martinez C, Benitez J. Interethnic differences in drug metabolism: influence of genetic and environmental factors on debrisoquine hydroxylation phenotype. Eur J Drug Metab Pharmacokin. 1996;21:129–138. - PubMed
-
- Xie HG, Huang SL, Xu Z, Xiao ZS, He N, Zhou HH. Evidence for the effect of gender on activity of (S) mephenytoin 4′-hydroxylase (CYP2C19) in a Chinese population. Pharmacogenetics. 1997;7:115–119. - PubMed
-
- Tamminga WJ, Wemer J, Oosterhuis B, et al. CYP2D6 and CYP2C19 activity in a large population of Dutch healthy volunteers: indications for oral contraceptive-related gender differences. Eur J Clin Pharmacol. 1999;55:177–184. 10.1007/s002280050615. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

