Efficacy of cytokine gene transfection may differ for autologous and allogeneic tumour cell vaccines
- PMID: 11260324
- PMCID: PMC1783163
- DOI: 10.1046/j.1365-2567.2001.01176.x
Efficacy of cytokine gene transfection may differ for autologous and allogeneic tumour cell vaccines
Abstract
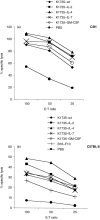
Whole tumour cells are a logical basis for generating immunity against the cancers they comprise or represent. A number of human trials have been initiated using cytokine-transfected whole tumour cells of autologous (patient-derived) or allogeneic [major histocompatibility complex (MHC)-disparate] origin as vaccines. Although precedent exists for the efficacy of autologous-transfected cell vaccines in animal models, little preclinical evidence confirms that these findings will extrapolate to allogeneic-transfected cell vaccines. In order to address this issue a murine melanoma cell line (K1735) was transfected to secrete interleukin (IL)-2, IL-4, IL-7 or granulocyte-macrophage colony-stimulating factor (GM-CSF); cytokines currently in use in trials. The efficacy of these cells as irradiated vaccines was tested head-to-head in syngeneic (C3H) mice and in MHC-disparate (C57BL/6) mice, the former being subsequently challenged with K1735 cells and the latter with naturally cross-reactive B16-F10 melanoma cells. Whilst the GM-CSF-secreting vaccine was the most effective at generating protection in C3H mice, little enhancement in protection above the wild-type vaccine was seen with any of the transfections for the allogeneic vaccines, even though the wild-type vaccine was more effective than the autologous B16-F10 vaccine. Anti-tumour cytotoxic T-lymphocyte (CTL) activity was detected in both models but did not correlate well with protection, whilst in vitro anti-tumour interferon-gamma (IFN-gamma) secretion tended to be higher following the GM-CSF-secreting vaccine. Cytokine transfection of vaccines generally increased anti-tumour CTL activity and IFN-gamma secretion (T helper type 1 response). Further studies in other model systems are required to confirm this apparent lack of benefit of cytokine transduction over wild-type allogeneic vaccines, and to determine which in vitro assays will correlate best with protection in vivo.
Figures




References
-
- Cavallo F, Di Pierro F, Giovarelli M, et al. Protective and curative potential of vaccination with interleukin-2-gene-transfected cells from a spontaneous mouse mammary adenocarcinoma. Cancer Res. 1993;53:5067–70. - PubMed
-
- Golumbek PT, Lazenby AJ, Levitsky HI, Jaffee LM, Karasuyama H, Baker M, Pardoll DM. Treatment of established renal cancer by tumor cells engineered to secrete interleukin-4. Science. 1991;254:713–16. - PubMed
-
- Allione A, Consalvo M, Nanni P, et al. Immunizing and curative potential of replicating and nonreplicating murine mammary adenocarcinoma cells engineered with interleukin (IL)-2, IL-4, IL-6, IL-7, IL-10, tumor necrosis factor alpha, granulocyte-macrophage colony-stimulating factor, and gamma-interferon gene or admixed with conventional adjuvants. Cancer Res. 1994;54:6022–6. - PubMed
-
- Townsend SE, Allison JP. tumor rejection after direct costimulation of CD8+ t cells by B7-transfected melanoma cells. Science. 1993;259:368–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

