Xenopus tropicalis oocytes as an advantageous model system for the study of intracellular Ca(2+) signalling
- PMID: 11264232
- PMCID: PMC1572681
- DOI: 10.1038/sj.bjp.0703922
Xenopus tropicalis oocytes as an advantageous model system for the study of intracellular Ca(2+) signalling
Abstract
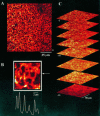
1. The purpose of this study was to compare oocytes from the pipid frogs Xenopus tropicalis and Xenopus laevis, with respect to their utility for studying Ca(2+) signalling mechanisms and for expression of heterologous proteins. 2. We show that X. tropicalis oocytes possess an intracellular Ca(2+) store that is mobilized by inositol (1,4,5) trisphosphate (IP(3)). Ca(2+) signalling is activated by endogenous lysophosphatidic acid receptors and cytosolic Ca(2+) activates a plasma membrane chloride conductance. The spatiotemporal organization of cytosolic Ca(2+) signals, from the microscopic architecture of elementary Ca(2+) 'puffs' to the macroscopic patterns of Ca(2+) spiking are closely similar to the local and global patterns of Ca(2+) release previously characterized in oocytes from X. laevis. 3. By injecting X. tropicalis oocytes with cDNA encoding an ER-targeted fluorescent protein construct, we demonstrate the capacity of the X. tropicalis oocyte to readily express heterologous proteins. The organization of ER is polarized across the oocyte, with the IP(3)-releaseable store targeted within an approximately 8 microm wide band that circumscribes the cell. 4. We conclude that the X. tropicalis oocyte shares many of the characteristics that have made oocytes of X. laevis a favoured system for studying Ca(2+) signalling mechanisms. Moreover, X. tropicalis oocytes display further practical advantages in terms of imaging depth, Ca(2+) signal magnitude and electrical properties. These further enhance the appeal of X. tropicalis as an experimental system, in addition to its greater amenability to transgenic approaches.
Figures







References
-
- AARHUS R., GEE K., LEE H.C. Caged cyclic ADP-ribose. J. Biol. Chem. 1995;270:7745–7749. - PubMed
-
- AMAYA E., OFFIELD M.F., GRAINGER R.M. Frog genetics: Xenopus tropicalis jumps into the future. Trends Genet. 1998;14:253–255. - PubMed
-
- BEREITER-HAHN J., VÖTH M. Dynamics of mitochondria in living cells: shape changes, dislocations, fusion, and fission of mitochondria. Microscopy Res. Technique. 1994;27:198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

