Cyclo-oxygenase and lipoxygenase pathways in mast cell dependent-neurogenic inflammation induced by electrical stimulation of the rat saphenous nerve
- PMID: 11264253
- PMCID: PMC1572691
- DOI: 10.1038/sj.bjp.0703950
Cyclo-oxygenase and lipoxygenase pathways in mast cell dependent-neurogenic inflammation induced by electrical stimulation of the rat saphenous nerve
Abstract
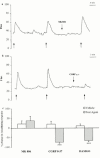
1. We investigated the role of arachidonic acid metabolism and assessed the participation of mast cells and leukocytes in neurogenic inflammation in rat paw skin. We compared the effect of lipoxygenase (LOX) and cyclo-oxygenase (COX) inhibitors on oedema induced by saphenous nerve stimulation, substance P (SP), and compound 48/80. 2. Intravenous (i.v.) pre-treatment with a dual COX/LOX inhibitor (RWJ 63556), a dual LOX inhibitor/cysteinyl-leukotriene (CysLt) receptor antagonist (Rev 5901), a LOX inhibitor (AA 861), a five-lipoxygenase activating factor (FLAP) inhibitor (MK 886), or a glutathione S-transferase inhibitor (ethacrynic acid) significantly inhibited (40 to 60%) the development of neurogenic oedema, but did not affect cutaneous blood flow. Intradermal (i.d.) injection of LOX inhibitors reduced SP-induced oedema (up to 50% for RWJ 63556 and MK 886), whereas ethacrynic acid had a potentiating effect. 3. Indomethacin and rofecoxib, a highly selective COX-2 inhibitor, did not affect neurogenic and SP-induced oedema. Surprisingly, the structurally related COX-2 inhibitors, NS 398 and nimesulide, significantly reduced both neurogenic and SP-induced oedema (70% and 42% for neurogenic oedema, respectively; 49% and 46% for SP-induced oedema, respectively). 4. COX-2 mRNA was undetectable in saphenous nerves and paw skin biopsy samples, before and after saphenous nerve stimulation. 5. A mast cell stabilizer, cromolyn, and a H(1) receptor antagonist, mepyramine, significantly inhibited neurogenic (51% and 43%, respectively) and SP-induced oedema (67% and 63%, respectively). 6. The co-injection of LOX inhibitors and compound 48/80 did not alter the effects of compound 48/80. Conversely, ethacrynic acid had a significant potentiating effect. The pharmacological profile of the effect of COX inhibitors on compound 48/80-induced oedema was similar to that of neurogenic and SP-induced oedema. 7. The polysaccharide, fucoidan (an inhibitor of leukocyte rolling) did not affect neurogenic or SP-induced oedema. 8. Thus, (i) SP-induced leukotriene synthesis is involved in the development of neurogenic oedema in rat paw skin; (ii) this leukotriene-mediated plasma extravasation might be independent of mast cell activation and/or of the adhesion of leukocytes to the endothelium; (iii) COX did not appear to play a significant role in this process.
Figures



References
-
- BALUK P., HIRATA A., THURSTON G., FUJIWARA T., NEAL C.R., MICHEL C.C., MCDONALD D.M. Endothelial gaps: time course of formation and closure in inflamed venules of rats. Am. J. Physiol. 1997;272:L155–L170. - PubMed
-
- BARBER A. μ- and κ-opioid receptor agonists produce peripheral inhibition of neurogenic plasma extravasation in rat skin. Eur. J. Pharmacol. 1993;236:113–120. - PubMed
-
- BEACH V.L., STEINETZ B.G. Quantitative measurement of Evans Blue space in the tissues of the rat: influence of 5-hydroxytryptamine antagonists and phenelzine on experimental inflammation. J. Pharmacol. Exp. Ther. 1961;131:400–406. - PubMed
-
- BELL R.L., HARRIS R.R. The enzymology and pharmacology of 5-lipoxygenase and 5-lipoxygenase activating protein. Clin. Rev. Allergy Immunol. 1999;17:91–109. - PubMed
-
- BRAIN S.D., MOORE P.K. Pain and Neurogenic Inflammation. Basel: Birkhäuser Verlag; 1999.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

