Neurofilaments are nonessential to the pathogenesis of toxicant-induced axonal degeneration
- PMID: 11264303
- PMCID: PMC6762395
- DOI: 10.1523/JNEUROSCI.21-07-02278.2001
Neurofilaments are nonessential to the pathogenesis of toxicant-induced axonal degeneration
Abstract
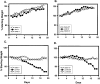
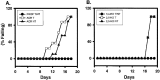
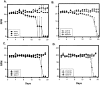
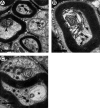
Axonal neurofilament (NF) accumulations occur before development of symptoms and before other pathological changes among idiopathic neurodegenerative diseases and toxic neuropathies, suggesting a cause-effect relationship. The dependence of symptoms and axonal degeneration on neurofilament accumulation has been tested here in a transgenic mouse model (Eyer and Peterson, 1994) lacking axonal NFs and using two prototypic toxicant models. Chronic acrylamide (ACR) or 2,5-hexanedione exposure resulted in progressive and cumulative increases in sensorimotor deficits. Neurobehavioral tests demonstrated similar expression of neurotoxicity in transgenic (T) mice and their nontransgenic (NT) littermates (containing normal numbers of axonal NFs). Axonal lesions were frequently observed after exposure to either toxicant. Quantitation of ACR-induced lesions demonstrated the distal location of pathology and equal susceptibility of T and NT axons. We conclude that axonal NFs have no effect on neurotoxicity and the pattern of pathology in these mammalian toxic neuropathies. These results also suggest that the role of neurofilament accumulation in the pathogenesis of neurodegenerative diseases requires careful evaluation.
Figures


Similar articles
-
Axonal neurofilaments are nonessential elements of toxicant-induced reductions in fast axonal transport: video-enhanced differential interference microscopy in peripheral nervous system axons.Toxicol Appl Pharmacol. 1999 Nov 15;161(1):50-8. doi: 10.1006/taap.1999.8780. Toxicol Appl Pharmacol. 1999. PMID: 10558923
-
Neurofilaments are non-essential elements of toxicant-induced reductions in fast axonal transport: pulse labeling in CNS neurons.Neurotoxicology. 2000 Aug;21(4):447-57. Neurotoxicology. 2000. PMID: 11022855
-
Toxic axonal degeneration occurs independent of neurofilament accumulation.J Neurosci Res. 1994 Oct 15;39(3):347-54. doi: 10.1002/jnr.490390312. J Neurosci Res. 1994. PMID: 7869427
-
Redefining toxic distal axonopathies.Toxicol Lett. 2000 Mar 15;112-113:23-33. doi: 10.1016/s0378-4274(99)00249-0. Toxicol Lett. 2000. PMID: 10720709 Review.
-
Toxic neuropathies: Mechanistic insights based on a chemical perspective.Neurosci Lett. 2015 Jun 2;596:78-83. doi: 10.1016/j.neulet.2014.08.054. Epub 2014 Sep 16. Neurosci Lett. 2015. PMID: 25218479 Free PMC article. Review.
Cited by
-
The Role of Protein Adduction in Toxic Neuropathies of Exogenous and Endogenous Origin.Toxics. 2021 Apr 29;9(5):98. doi: 10.3390/toxics9050098. Toxics. 2021. PMID: 33946924 Free PMC article. Review.
-
Probing mechanisms of axonopathy. Part I: Protein targets of 1,2-diacetylbenzene, the neurotoxic metabolite of aromatic solvent 1,2-diethylbenzene.Toxicol Sci. 2008 Sep;105(1):134-41. doi: 10.1093/toxsci/kfn103. Epub 2008 May 22. Toxicol Sci. 2008. PMID: 18502740 Free PMC article.
-
Spatio-temporal changes in neurofilament proteins immunoreactivity following kainate-induced cerebellar lesion in rats.Cell Mol Neurobiol. 2004 Jun;24(3):367-78. doi: 10.1023/b:cemn.0000022769.44211.2b. Cell Mol Neurobiol. 2004. PMID: 15206820 Free PMC article.
-
Gamma-diketone axonopathy: analyses of cytoskeletal motors and highways in CNS myelinated axons.Toxicol Sci. 2010 Sep;117(1):180-9. doi: 10.1093/toxsci/kfq176. Epub 2010 Jun 16. Toxicol Sci. 2010. PMID: 20554699 Free PMC article.
-
Review of the multiple aspects of neurofilament functions, and their possible contribution to neurodegeneration.Mol Neurobiol. 2008 Aug;38(1):27-65. doi: 10.1007/s12035-008-8033-0. Epub 2008 Jul 23. Mol Neurobiol. 2008. PMID: 18649148 Review.
References
-
- Anthony DC, Boekelheide K, Graham DG. The effect of 3,4-dimethyl substitution on the neurotoxicity of 2,5-hexanedione. I. Accelerated clinical neuropathy is accompanied by more proximal axonal swellings. Toxicol Appl Pharmacol. 1983a;71:362–371. - PubMed
-
- Anthony DC, Boekelheide K, Anderson CW, Graham DG. The effect of 3,4-dimethyl substitution on neurotoxicity of 2,5-hexanedione. II. Dimethyl substitution accelerates pyrrole formation and protein crosslinking. Toxicol Appl Pharmacol. 1983b;71:372–382. - PubMed
-
- Anthony DC, Giangaspero F, Graham DG. The spatio-temporal pattern of the axonopathy associated with the neurotoxicity of 3,4-dimethyl-2,5-hexanedione in the rat. J Neuropathol Exp Neurol. 1983c;42:548–560. - PubMed
-
- Bradley WG, Asbury AK. Radioautographic studies of Schwann cell behavior. I. Acrylamide neuropathy in the mouse. J Neuropathol Exp Neurol. 1991;29:500–506. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
