Changes in microtubule stability and density in myelin-deficient shiverer mouse CNS axons
- PMID: 11264304
- PMCID: PMC6762390
- DOI: 10.1523/JNEUROSCI.21-07-02288.2001
Changes in microtubule stability and density in myelin-deficient shiverer mouse CNS axons
Abstract
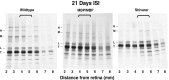
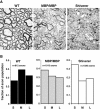
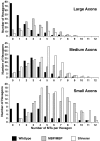
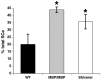
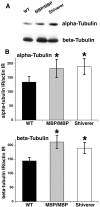
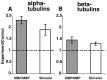
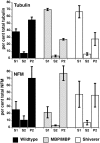
Altered axon-Schwann cell interactions in PNS myelin-deficient Trembler mice result in changed axonal transport rates, neurofilament and microtubule-associated protein phosphorylation, neurofilament density, and microtubule stability. To determine whether PNS and CNS myelination have equivalent effects on axons, neurofilaments, and microtubules in CNS, myelin-deficient shiverer axons were examined. The genetic defect in shiverer is a deletion in the myelin basic protein (MBP) gene, an essential component of CNS myelin. As a result, shiverer mice have little or no compact CNS myelin. Slow axonal transport rates in shiverer CNS axons were significantly increased, in contrast to the slowing in demyelinated PNS nerves. Even more striking were substantial changes in the composition and properties of microtubules in shiverer CNS axons. The density of axonal microtubules is increased, reflecting increased expression of tubulin in shiverer, and the stability of microtubules is drastically reduced in shiverer axons. Shiverer transgenic mice with two copies of a wild-type myelin basic protein transgene have an intermediate level of compact myelin, making it possible to determine whether the actual level of compact myelin is an important regulator of axonal microtubules. Both increased microtubule density and reduced microtubule stability were still observed in transgenic mouse nerves, indicating that signals beyond synaptogenesis and the mere presence of compact myelin are required for normal regulation of the axonal microtubule cytoskeleton.
Figures
Similar articles
-
Modulation of the axonal microtubule cytoskeleton by myelinating Schwann cells.J Neurosci. 1994 Dec;14(12):7440-50. doi: 10.1523/JNEUROSCI.14-12-07440.1994. J Neurosci. 1994. PMID: 7996186 Free PMC article.
-
Formation of compact myelin is required for maturation of the axonal cytoskeleton.J Neurosci. 1999 Sep 1;19(17):7278-88. doi: 10.1523/JNEUROSCI.19-17-07278.1999. J Neurosci. 1999. PMID: 10460234 Free PMC article.
-
Altered slow axonal transport and regeneration in a myelin-deficient mutant mouse: the trembler as an in vivo model for Schwann cell-axon interactions.J Neurosci. 1990 Jun;10(6):1855-65. doi: 10.1523/JNEUROSCI.10-06-01855.1990. J Neurosci. 1990. PMID: 2355253 Free PMC article.
-
The dysmyelinating mouse mutations shiverer (shi) and myelin deficient (shimld).Behav Genet. 1990 Mar;20(2):213-34. doi: 10.1007/BF01067791. Behav Genet. 1990. PMID: 1693848 Review.
-
Myelin-associated glycoprotein is a myelin signal that modulates the caliber of myelinated axons.J Neurosci. 1998 Mar 15;18(6):1953-62. doi: 10.1523/JNEUROSCI.18-06-01953.1998. J Neurosci. 1998. PMID: 9482781 Free PMC article. Review.
Cited by
-
Developmental axon diameter growth of central nervous system axons does not depend on ensheathment or myelination by oligodendrocytes.bioRxiv [Preprint]. 2025 Jan 10:2025.01.10.632348. doi: 10.1101/2025.01.10.632348. bioRxiv. 2025. PMID: 39829751 Free PMC article. Preprint.
-
Myelin associated glycoprotein cross-linking triggers its partitioning into lipid rafts, specific signaling events and cytoskeletal rearrangements in oligodendrocytes.Neuron Glia Biol. 2004 Feb;1(1):35-46. doi: 10.1017/s1740925x04000067. Neuron Glia Biol. 2004. PMID: 16998591 Free PMC article.
-
Magnetic resonance imaging of myelin.Neurotherapeutics. 2007 Jul;4(3):460-84. doi: 10.1016/j.nurt.2007.05.004. Neurotherapeutics. 2007. PMID: 17599712 Free PMC article. Review.
-
The Axon-Myelin Unit in Development and Degenerative Disease.Front Neurosci. 2018 Jul 11;12:467. doi: 10.3389/fnins.2018.00467. eCollection 2018. Front Neurosci. 2018. PMID: 30050403 Free PMC article. Review.
-
A Role of Microtubules in Oligodendrocyte Differentiation.Int J Mol Sci. 2020 Feb 5;21(3):1062. doi: 10.3390/ijms21031062. Int J Mol Sci. 2020. PMID: 32033476 Free PMC article.
References
-
- Aguayo A, David S, Bray G. Influence of the glial environment on the elongation of axons after injury: transplantation studies in adult rodents. J Exp Biol. 1981;95:231–240. - PubMed
-
- Benley M, Aguayo A. Extensive elongation of axons from rat brain into peripheral nerve graft. Nature. 1982;296:150–152. - PubMed
-
- Brady S. Axonal transport methods and applications. In: Boulton A, Baker G, editors. Neuromethods, general neurochemical techniques. Humana; Clifton, NJ: 1985. pp. 419–476.
-
- Brady ST. Increases in cold-insoluble tubulin during aging. Soc Neurosci Abstr. 1984;10:273.
-
- Brady ST. Cytotypic specializations of the neuronal cytoskeleton and cytomatrix: implications for neuronal growth and regeneration. In: Haber B, Gorio A, Vellis JD, Perez-Polo JR, editors. Cellular and molecular aspects of neural development and regeneration. Springer; New York: 1988. pp. 311–322.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
