A depletable pool of adenosine in area CA1 of the rat hippocampus
- PMID: 11264305
- PMCID: PMC6762415
- DOI: 10.1523/JNEUROSCI.21-07-02298.2001
A depletable pool of adenosine in area CA1 of the rat hippocampus
Abstract
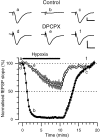
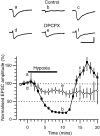
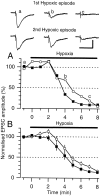
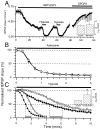
Adenosine plays a major modulatory and neuroprotective role in the mammalian CNS. During cerebral metabolic stress, such as hypoxia or ischemia, the increase in extracellular adenosine inhibits excitatory synaptic transmission onto vulnerable neurons via presynaptic adenosine A(1) receptors, thereby reducing the activation of postsynaptic glutamate receptors. Using a combination of extracellular and whole-cell recordings in the CA1 region of hippocampal slices from 12- to 24-d-old rats, we have found that this protective depression of synaptic transmission weakens with repeated exposure to hypoxia, thereby allowing potentially damaging excitation to both persist for longer during oxygen deprivation and recover more rapidly on reoxygenation. This phenomenon is unlikely to involve A(1) receptor desensitization or impaired nucleoside transport. Instead, by using the selective A(1) antagonist 8-cyclopentyl-1,3-dipropylxanthine and a novel adenosine sensor, we demonstrate that adenosine production is reduced with repeated episodes of hypoxia. Furthermore, this adenosine depletion can be reversed at least partially either by the application of exogenous adenosine, but not by a stable A(1) agonist, N(6)-cyclopentyladenosine, or by endogenous means by prolonged (2 hr) recovery between hypoxic episodes. Given the vital neuroprotective role of adenosine, these findings suggest that depletion of adenosine may underlie the increased neuronal vulnerability to repetitive or secondary hypoxia/ischemia in cerebrovascular disease and head injury.
Figures




Similar articles
-
Brief, repeated, oxygen-glucose deprivation episodes protect neurotransmission from a longer ischemic episode in the in vitro hippocampus: role of adenosine receptors.Br J Pharmacol. 2003 Sep;140(2):305-14. doi: 10.1038/sj.bjp.0705442. Epub 2003 Aug 11. Br J Pharmacol. 2003. PMID: 12970092 Free PMC article.
-
Putative depolarisation-induced retrograde signalling accelerates the repeated hypoxic depression of excitatory synaptic transmission in area CA1 of rat hippocampus via group I metabotropic glutamate receptors.Neuroscience. 2012 Oct 11;222:159-72. doi: 10.1016/j.neuroscience.2012.07.034. Epub 2012 Jul 27. Neuroscience. 2012. PMID: 22842516
-
High-resolution real-time recording with microelectrode biosensors reveals novel aspects of adenosine release during hypoxia in rat hippocampal slices.J Neurochem. 2003 Sep;86(6):1506-15. doi: 10.1046/j.1471-4159.2003.01957.x. J Neurochem. 2003. PMID: 12950459
-
Cellular physiology of hypoxia of the mammalian central nervous system.Res Publ Assoc Res Nerv Ment Dis. 1993;71:51-65. Res Publ Assoc Res Nerv Ment Dis. 1993. PMID: 8380239 Review.
-
Plasticity of purine release during cerebral ischemia: clinical implications?J Cell Mol Med. 2003 Oct-Dec;7(4):362-75. doi: 10.1111/j.1582-4934.2003.tb00239.x. J Cell Mol Med. 2003. PMID: 14754505 Free PMC article. Review.
Cited by
-
Point-of-care measurements reveal release of purines into venous blood of stroke patients.Purinergic Signal. 2019 Jun;15(2):237-246. doi: 10.1007/s11302-019-09647-4. Epub 2019 Mar 12. Purinergic Signal. 2019. PMID: 30859371 Free PMC article.
-
Intracellular ATP influences synaptic plasticity in area CA1 of rat hippocampus via metabolism to adenosine and activity-dependent activation of adenosine A1 receptors.J Neurosci. 2011 Apr 20;31(16):6221-34. doi: 10.1523/JNEUROSCI.4039-10.2011. J Neurosci. 2011. PMID: 21508245 Free PMC article.
-
Comparative analysis of adenosine 1 receptor expression and function in hippocampal and hypothalamic neurons.Inflamm Res. 2025 Jan 8;74(1):11. doi: 10.1007/s00011-024-01980-8. Inflamm Res. 2025. PMID: 39775928 Free PMC article.
-
Deletion of presynaptic adenosine A1 receptors impairs the recovery of synaptic transmission after hypoxia.Neuroscience. 2005;132(3):575-80. doi: 10.1016/j.neuroscience.2004.12.009. Neuroscience. 2005. PMID: 15837119 Free PMC article.
-
Adenosine release in nucleus tractus solitarii does not appear to mediate hypoxia-induced respiratory depression in rats.J Physiol. 2002 Oct 1;544(Pt 1):161-70. doi: 10.1113/jphysiol.2002.024174. J Physiol. 2002. PMID: 12356889 Free PMC article.
References
-
- Arlinghaus L, Lee KS. Endogenous adenosine mediates the sustained inhibition of excitatory synaptic transmission during moderate hypoxia. Brain Res. 1996;724:265–268. - PubMed
-
- Berne RM, Rubio R, Curran GH. Release of adenosine from ischemic brain. Circ Res. 1974;35:262–271.
-
- Blumbergs PC. Pathology. In: Reilly P, Bullock R, editors. Head injury: pathophysiology and management of severe closed injury. Chapman & Hall; London: 1997. pp. 40–70.
-
- Chen J, Simon R. Ischemic tolerance in the brain. Neurology. 1997;48:306–311. - PubMed
-
- Cherubini E, Ben-Ari Y, Krnjevic K. Anoxia produces smaller changes in synaptic transmission, membrane potential, and input resistance in immature rat hippocampus. J Neurophysiol. 1989;62:882–895. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
