Synapse formation is arrested in retinal photoreceptors of the zebrafish nrc mutant
- PMID: 11264308
- PMCID: PMC6762396
- DOI: 10.1523/JNEUROSCI.21-07-02330.2001
Synapse formation is arrested in retinal photoreceptors of the zebrafish nrc mutant
Abstract
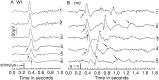
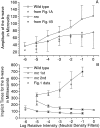
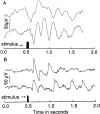
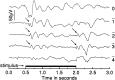
We describe the effects of a recessive mutation on visual behavior, the electroretinogram (ERG), and photoreceptor structure in zebrafish. At 6 d post-fertilization (dpf), no optokinetic reflex could be elicited in no optokinetic response c (nrc) mutant animals under any test condition. The animals exhibited ERG responses at 5-7 dpf that were markedly abnormal and could be categorized into two groups. The first showed an initial negative a-wave followed by a delayed positive b-wave of small amplitude. Often a second ERG-like response was recorded after the initial b-wave. The second group showed only a large negative a-wave; an initial b-wave was not evident. In most recordings additional oscillatory waves varying in number, amplitude, and time course were observed. Multiple responses at the cessation of long-duration flashes were also observed. Light and electron microscopy revealed that the cone photoreceptor pedicles of nrc fish were highly abnormal. Although the appropriate number of synaptic ribbons formed in these terminals, they "floated" in the terminal, unassociated with postsynaptic processes or arciform densities. The few processes invaginating the nrc pedicles resembled those of horizontal cells. Invaginating bipolar cell processes were rare, but basal contacts were observed on pedicle surfaces. The severity of the mutation did not change between 6 and 8 dpf, showing that there is neither a delay in development nor a degeneration of the terminals; rather, nrc pedicle development appears arrested. Bipolar cell terminals in the inner plexiform layer made normal ribbon synapses; thus, the mutation appears to affect only the outer retina.
Figures





References
-
- Blanks JC, Adinolfi AM, Lolley RN. Synaptogenesis in the photoreceptor terminal of the mouse retina. J Comp Neurol. 1974;156:81–94. - PubMed
-
- Braekevelt CR. Retinal pigment epithelium fine structure in the velvet cichlid (Astronotus ocellatus). Anat Embryol. 1992;186:371–377. - PubMed
-
- Bunt AH. Enzymatic digestion of synaptic ribbons in amphibian retinal photoreceptors. Brain Res. 1971;25:571–577. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
