Formation and function of synapses with respect to Schwann cells at the end of motor nerve terminal branches on mature amphibian (Bufo marinus) muscle
- PMID: 11264312
- PMCID: PMC6762398
- DOI: 10.1523/JNEUROSCI.21-07-02380.2001
Formation and function of synapses with respect to Schwann cells at the end of motor nerve terminal branches on mature amphibian (Bufo marinus) muscle
Abstract
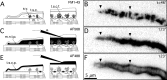
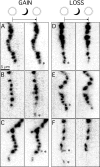
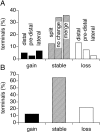
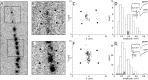
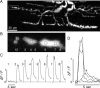
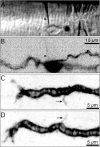
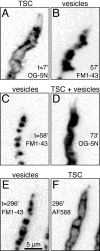
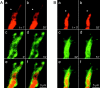
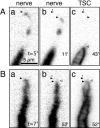
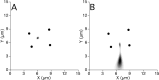
A study has been made of the formation and regression of synapses with respect to Schwann cells at the ends of motor nerve terminal branches in mature toad (Bufo marinus) muscle. Synapse formation and regression, as inferred from the appearance and loss of N-(3-triethylammoniumpropyl)-4-(4-(dibutylamino)styryl) pyridinium dibromide (FM1-43)-stained vesicle clusters, occurred at the ends of terminal branches over a 16 hr period. Multiple microelectrodes placed in an array about FM1-43 blobs at the ends of terminal branches detected the electrical signs of neurotransmitter being released onto receptors. Injection of a calcium indicator (Oregon Green 488 BAPTA-1) into the motor nerve with subsequent imaging of the calcium transients, in response to stimulation, often showed a reduced calcium influx in the ends of terminal branches. Injection of a fluorescent dye into motor nerves revealed the full extent of their terminal branches and growing processes. Injection of the terminal Schwann cells (TSCs) often revealed pseudopodial TSC processes up to 10-microm-long. Imaging of these TSC processes over minutes or hours showed that they were highly labile and capable of extending several micrometers in a few minutes. Injection of motor nerve terminals with a different dye to that injected into their TSCs revealed that terminal processes sometimes followed the TSC processes over a few hours. It is suggested that the ends of motor nerve terminals in vivo are in a constant state of remodeling through the formation and regression of processes, that TSC processes guide the remodeling, and that it can occur over a relatively short period of time.
Figures

Similar articles
-
Schwann cell dynamics with respect to newly formed motor-nerve terminal branches on mature (Bufo marinus) muscle fibers.J Neurocytol. 2003 May;32(4):381-92. doi: 10.1023/B:NEUR.0000011332.96472.b2. J Neurocytol. 2003. PMID: 14724381
-
Vesicle-associated proteins and quantal release at single active zones of amphibian (Bufo marinus) motor-nerve terminals.J Neurophysiol. 1999 Sep;82(3):1133-46. doi: 10.1152/jn.1999.82.3.1133. J Neurophysiol. 1999. PMID: 10482733
-
Quantal secretion at release sites of nerve terminals in toad (Bufo marinus) muscle during formation of topographical maps.J Physiol. 1988 Jul;401:567-79. doi: 10.1113/jphysiol.1988.sp017180. J Physiol. 1988. PMID: 2902220 Free PMC article.
-
The formation and function of single transmitter release sites at mature amphibian motor-nerve terminals.J Neurocytol. 2003 Jun-Sep;32(5-8):447-72. doi: 10.1023/B:NEUR.0000020604.21826.41. J Neurocytol. 2003. PMID: 15034247 Review.
-
Schwann cells participate in synapse elimination at the developing neuromuscular junction.Curr Opin Neurobiol. 2017 Dec;47:176-181. doi: 10.1016/j.conb.2017.10.010. Epub 2017 Nov 6. Curr Opin Neurobiol. 2017. PMID: 29121585 Free PMC article. Review.
Cited by
-
Purinergic junctional transmission and propagation of calcium waves in spinal cord astrocyte networks.Biophys J. 2006 Nov 1;91(9):3560-71. doi: 10.1529/biophysj.106.082073. Epub 2006 Aug 11. Biophys J. 2006. PMID: 16905605 Free PMC article.
-
Characteristics of calcium transient in different parts of frog nerve terminal in response to nerve impulse.Dokl Biol Sci. 2010 Mar-Apr;431:83-5. doi: 10.1134/s0012496610020043. Dokl Biol Sci. 2010. PMID: 20506840 No abstract available.
-
Mechanisms and roles of axon-Schwann cell interactions.J Neurosci. 2004 Oct 20;24(42):9250-60. doi: 10.1523/JNEUROSCI.3649-04.2004. J Neurosci. 2004. PMID: 15496660 Free PMC article. Review. No abstract available.
-
Clinical relevance of terminal Schwann cells: An overlooked component of the neuromuscular junction.J Neurosci Res. 2018 Jul;96(7):1125-1135. doi: 10.1002/jnr.24231. Epub 2018 Mar 13. J Neurosci Res. 2018. PMID: 29536564 Free PMC article. Review.
-
Perisynaptic Schwann Cells at the Neuromuscular Synapse: Adaptable, Multitasking Glial Cells.Cold Spring Harb Perspect Biol. 2015 Aug 20;7(10):a020503. doi: 10.1101/cshperspect.a020503. Cold Spring Harb Perspect Biol. 2015. PMID: 26430218 Free PMC article. Review.
References
-
- Ahmari SE, Buchanan J, Smith SJ. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat Neurosci. 2000;3:445–451. - PubMed
-
- Astrow SH, Qiang H, Ko CP. Perisynaptic Schwann cells at neuromuscular junctions revealed by a novel monoclonal antibody. J Neurocytol. 1998;27:667–681. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
