Postlearning consolidation of birdsong: stabilizing effects of age and anterior forebrain lesions
- PMID: 11264324
- PMCID: PMC6762407
- DOI: 10.1523/JNEUROSCI.21-07-02501.2001
Postlearning consolidation of birdsong: stabilizing effects of age and anterior forebrain lesions
Abstract
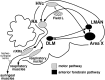
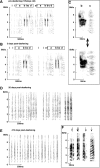
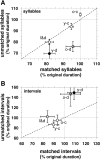
Birdsong is a learned, sequenced motor skill. For the zebra finch, learned song normally remains unchanging beyond early adulthood. However, stable adult song will gradually deteriorate after deafening (Nordeen and Nordeen, 1992), indicating an ongoing influence of auditory feedback on learned song. This plasticity of adult song in response to deafening gradually declines with age (Lombardino and Nottebohm, 2000), suggesting that, after song learning, there continue to be changes in the brain that progressively stabilize the song motor program. A qualitatively similar stabilization of learned song can be precipitated artificially by lesions of a basal ganglia circuit in the songbird anterior forebrain (Brainard and Doupe, 2000), raising the question of whether and how these two forms of song stabilization are related. We investigated this issue by characterizing the deterioration of song that occurs after deafening in young adult birds and the degree to which that deterioration is reduced by age or by lesions of the anterior forebrain that were directed at the lateral portion of the magnocellular nucleus of the anterior neostriatum (LMAN). In most respects, LMAN lesions stabilized song to a significantly greater extent than did aging; whereas old-deafened birds eventually exhibited significant deterioration of song, lesioned-deafened birds generally did not differ from controls. The one exception was for song tempo, which was significantly stabilized by age, but not by LMAN lesions. The results indicate that LMAN lesions do not simply mimic a normal aging process, and likewise suggest that the anterior forebrain pathway continues to play a role even in the residual song plasticity that is observed after the age-dependent stabilization of song.
Figures










References
-
- Ackermann H, Konczak J, Hertrich I. The temporal control of repetitive articulatory movements in Parkinson's disease. Brain Lang. 1997;56:312–319. - PubMed
-
- Arnold AP. The effects of castration on song development in zebra finches (Poephila guttata). J Exp Zool. 1975;191:261–278. - PubMed
-
- Basham ME, Nordeen EJ, Nordeen KW. Blockade of NMDA receptors in the anterior forebrain impairs sensory acquisition in the zebra finch. Neurobiol Learn Mem. 1996;66:295–304. - PubMed
-
- Bolhuis JJ. Mechanisms of avian imprinting: a review. Biol Rev Camb Philos Soc. 1991;66:303–345. - PubMed
-
- Bottjer SW, Hewer SJ. Castration and antisteroid treatment impair vocal learning in male zebra finches. J Neurobiol. 1992;23:337–353. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
