Basolateral amygdala-nucleus accumbens interactions in mediating glucocorticoid enhancement of memory consolidation
- PMID: 11264325
- PMCID: PMC6762383
- DOI: 10.1523/JNEUROSCI.21-07-02518.2001
Basolateral amygdala-nucleus accumbens interactions in mediating glucocorticoid enhancement of memory consolidation
Abstract
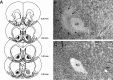
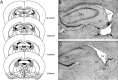
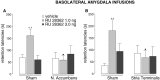
Systemic or intracerebral administration of glucocorticoids enhances memory consolidation in several tasks. Previously, we reported that these effects depend on an intact basolateral nucleus of the amygdala (BLA) and efferents from the BLA that run through the stria terminalis (ST). The BLA projects directly to the nucleus accumbens (NAc) via this ST pathway. The NAc also receives direct projections from the hippocampus and, therefore, may be a site of convergence of BLA and hippocampal influences in modulating memory consolidation. In support of this view, we found previously that lesions of either the NAc or the ST also block the memory-modulatory effect of systemically administered glucocorticoids. The present experiments examined the effects of lesions of the NAc or the ST on the memory-modulatory effects of intracerebral glucocorticoids on inhibitory avoidance training. Microinfusions of the specific glucocorticoid receptor agonist 11beta,17beta-dihydroxy-6,21-dimethyl-17alpha-pregna-4,6-trien-20yn-3-one (RU 28362; 1.0 or 3.0 ng) into either the BLA or the hippocampus of male Sprague Dawley rats administered immediately after training enhanced the 48 hr retention performance in a dose-dependent manner. Bilateral lesions of the NAc or the ST alone did not affect retention performance but blocked the memory enhancement induced by intra-BLA or intrahippocampal glucocorticoid receptor agonist administration. These findings indicate that the BLA-NAc pathway plays an essential role in mediating glucocorticoid effects on memory consolidation and suggest that the BLA interacts with hippocampal effects on memory consolidation via this pathway.
Figures


Similar articles
-
Glucocorticoid effects on memory consolidation depend on functional interactions between the medial prefrontal cortex and basolateral amygdala.J Neurosci. 2009 Nov 11;29(45):14299-308. doi: 10.1523/JNEUROSCI.3626-09.2009. J Neurosci. 2009. PMID: 19906977 Free PMC article.
-
Involvement of a basolateral amygdala complex-nucleus accumbens pathway in glucocorticoid-induced modulation of memory consolidation.Eur J Neurosci. 2000 Jan;12(1):367-75. doi: 10.1046/j.1460-9568.2000.00911.x. Eur J Neurosci. 2000. PMID: 10651892
-
Glucocorticoid receptor activation in the rat nucleus of the solitary tract facilitates memory consolidation: involvement of the basolateral amygdala.Eur J Neurosci. 1999 Apr;11(4):1317-23. doi: 10.1046/j.1460-9568.1999.00537.x. Eur J Neurosci. 1999. PMID: 10103127
-
1999 Curt P. Richter award. Glucocorticoids and the regulation of memory consolidation.Psychoneuroendocrinology. 2000 Apr;25(3):213-38. doi: 10.1016/s0306-4530(99)00058-x. Psychoneuroendocrinology. 2000. PMID: 10737694 Review.
-
Systems mediating acute glucocorticoid effects on memory consolidation and retrieval.Prog Neuropsychopharmacol Biol Psychiatry. 2003 Dec;27(8):1213-23. doi: 10.1016/j.pnpbp.2003.09.015. Prog Neuropsychopharmacol Biol Psychiatry. 2003. PMID: 14659476 Review.
Cited by
-
Stress administered prior to encoding impairs neutral but enhances emotional long-term episodic memories.Learn Mem. 2007 Dec 17;14(12):861-8. doi: 10.1101/lm.743507. Print 2007 Dec. Learn Mem. 2007. PMID: 18086830 Free PMC article.
-
Memory modulation.Behav Neurosci. 2011 Dec;125(6):797-824. doi: 10.1037/a0026187. Behav Neurosci. 2011. PMID: 22122145 Free PMC article. Review.
-
Gene-environment interplay in affect and dementia: emotional modulation of cognitive expression in personal outcomes.Neurotox Res. 2004;6(3):159-73. doi: 10.1007/BF03033219. Neurotox Res. 2004. PMID: 15325956
-
Nutraceutical Interventions for Post-Traumatic Stress Disorder in Animal Models: A Focus on the Hypothalamic-Pituitary-Adrenal Axis.Pharmaceuticals (Basel). 2022 Jul 20;15(7):898. doi: 10.3390/ph15070898. Pharmaceuticals (Basel). 2022. PMID: 35890196 Free PMC article. Review.
-
Emotional Modulation of Learning and Memory: Pharmacological Implications.Pharmacol Rev. 2017 Jul;69(3):236-255. doi: 10.1124/pr.116.013474. Pharmacol Rev. 2017. PMID: 28420719 Free PMC article. Review.
References
-
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–146. - PubMed
-
- Bermudez-Rattoni F, Introini-Collison I, Coleman-Mesches K, McGaugh JL. Insular cortex and amygdala lesions induced after aversive training impair retention: effects of degree of training. Neurobiol Learn Mem. 1997;67:57–63. - PubMed
-
- Cabib S, Castellano C, Patacchioli FR, Cigliana G, Angelucci L, Puglisi-Allegra S. Opposite strain-dependent effects of post-training corticosterone in a passive avoidance task in mice: role of dopamine. Brain Res. 1996;729:110–118. - PubMed
-
- Cahill L, McGaugh JL. Mechanisms of emotional arousal and lasting declarative memory. Trends Neurosci. 1998;21:294–299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
