Two functionally distinct forms of a retroviral receptor explain the nonreciprocal receptor interference among subgroups B, D, and E avian leukosis viruses
- PMID: 11264341
- PMCID: PMC114843
- DOI: 10.1128/JVI.75.8.3520-3526.2001
Two functionally distinct forms of a retroviral receptor explain the nonreciprocal receptor interference among subgroups B, D, and E avian leukosis viruses
Abstract
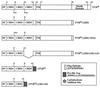
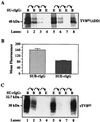
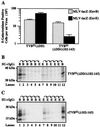
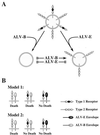
Subgroups B, D, and E avian leukosis viruses (ALV-B, -D, and -E) share the same chicken receptor, TVB(S1), a tumor necrosis factor receptor (TNFR)-related protein. These viruses, however, exhibit nonreciprocal receptor interference (NRI): cells preinfected with ALV-B or ALV-D are resistant to superinfection by viruses of all three subgroups, whereas those pre-infected by ALV-E are resistant only to superinfection by other subgroup E viruses. In this study, we investigated the basis of this phenomenon by characterizing the interaction of TVB(S1) with ALV-B Env or ALV-E Env. Sequential immunoprecipitation analysis using surface envelope immunoglobulin fusion proteins revealed the existence of two separate types of TVB(S1) that are encoded by the same cDNA clone. One form, designated the type 1 receptor, is specific for ALV-B and ALV-E. The other form, the type 2 receptor, is specific for ALV-B. We show that a protein consisting of only the first and second extracellular cysteine-rich domains of TVB(S1) is capable of forming both receptor types. However, the third extracellular cysteine-rich domain is required for efficient formation of the type 1 receptor. We also demonstrate that heterogeneous N-linked glycosylation cannot explain the difference in activities of the two receptor types. The existence of two types of TVB(S1) explains the NRI pattern between ALV-B and -E: subgroup B viruses establish receptor interference with both receptor types, whereas subgroup E viruses interfere only with the type 1 receptor, leaving the type 2 receptor available to mediate subsequent rounds of ALV-B entry. The formation of a TVB receptor type that is specific for cytopathic ALV may also have important implications for understanding how some subgroups of ALV cause cell death.
Figures

References
-
- Boeke J D, Stoye J P. Retrotransposons, endogenous retroviruses, and the evolution of retroelements. In: Coffin J M, Hughes S H, Varmus H E, editors. Retroviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 343–435. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

