AlaArg motif in the carboxyl terminus of the gamma(1)34.5 protein of herpes simplex virus type 1 is required for the formation of a high-molecular-weight complex that dephosphorylates eIF-2alpha
- PMID: 11264356
- PMCID: PMC114858
- DOI: 10.1128/JVI.75.8.3666-3674.2001
AlaArg motif in the carboxyl terminus of the gamma(1)34.5 protein of herpes simplex virus type 1 is required for the formation of a high-molecular-weight complex that dephosphorylates eIF-2alpha
Abstract
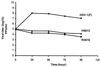
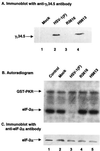
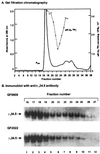
The gamma(1)34.5 protein of herpes simplex virus (HSV) type 1 functions to prevent the shutoff of protein synthesis mediated by the double-stranded-RNA-dependent protein kinase PKR. This is because gamma(1)34.5 associates with protein phosphatase 1 (PP1) through its carboxyl terminus, forming a high-molecular-weight complex that dephosphorylates the alpha subunit of translation initiation factor eIF-2 (eIF-2alpha). Here we show that Val193Glu and Phe195Leu substitutions in the PP1 signature motif of the gamma(1)34.5 protein abolished its ability to redirect PP1 to dephosphorylate eIF-2alpha and replication of mutant viruses was severely impaired. The gamma(1)34.5 protein, when expressed in Sf9 cells using a recombinant baculovirus, was capable of directing specific eIF-2alpha dephosphorylation. Deletions of amino acids 258 to 263 had no effect on activity of gamma(1)34.5. However, deletions of amino acids 238 to 258 abolished eIF-2alpha phosphatase activity but not PP1 binding activity. Interestingly, deletions in the AlaArg motif of the carboxyl terminus disrupted the high-molecular-weight complex that is required for dephosphorylation of eIF-2alpha. These results demonstrate that gamma(1)34.5 is functionally active in the absence of any other HSV proteins. In addition to a PP1 binding domain, the carboxyl terminus of gamma(1)34.5 contains an effector domain that is required to form a functional complex.
Figures





References
-
- Aitken A, Cohen P. Isolation and characterisation of active fragments of protein phosphatase inhibitor-1 from rabbit skeletal muscle. FEBS Lett. 1982;147:54–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

