Palindromic sequence plays a critical role in human foamy virus dimerization
- PMID: 11264362
- PMCID: PMC114864
- DOI: 10.1128/JVI.75.8.3731-3739.2001
Palindromic sequence plays a critical role in human foamy virus dimerization
Abstract
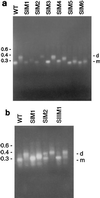
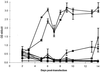
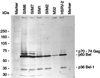
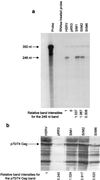
The retroviral RNA genome is dimeric, consisting of two identical strands of RNA linked near their 5' ends by a dimer linkage structure. Previously it was shown that human foamy virus (HFV) RNA transcribed in vitro contained three sites, designated SI, SII, and SIII, which contributed to the dimerization process (O. Erlwein, D. Cain, N. Fischer, A. Rethwilm, and M. O. McClure, Virology 229:251-258, 1997). To characterize these sites further, a series of mutants were designed and tested for their ability to dimerize in vitro. The primer binding site and a G tetrad in SI were dispensable for dimerization. However, a mutant that changed the 3' end of SI migrated slower on nondenaturing gels than wild-type RNA dimers. The sequence composition of the SII palindrome, consisting of 10 nucleotides, proved to be critical for in vitro dimerization, since mutations within this sequence or replacement of the sequence with a different palindrome of equal length impaired in vitro dimerization. The length of the palindrome also seems to play an important role. A moderate extension to 12 nucleotides was tolerated, whereas an extension to 16 nucleotides or more impaired dimerization. When nucleotides flanking the palindrome were mutated in a random fashion, dimerization was unaffected. Changing the SIII sequence also led to decreased dimer formation, confirming its contribution to the dimerization process. Interesting mutants were cloned into the infectious molecular clone of HFV, HSRV-2, and were transfected into BHK-21 cells. Mutations in SII that reduced dimerization in vitro also abolished virus replication. In contrast, constructs containing mutations in SI and SIII replicated to some extent in cell culture after an initial drop in viral replication. Analysis of the SIM1 mutant revealed reversion to the wild type but with the insertion of an additional two nucleotides. Analysis of cell-free virions demonstrated that both replication-competent and replication-defective mutants packaged nucleic acid. Thus, efficient dimerization is a critical step for HFV to generate infectious virus, but HFV RNA dimerization is not a prerequisite for packaging.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

