Y2, the smallest of the Sendai virus C proteins, is fully capable of both counteracting the antiviral action of interferons and inhibiting viral RNA synthesis
- PMID: 11264369
- PMCID: PMC114871
- DOI: 10.1128/JVI.75.8.3802-3810.2001
Y2, the smallest of the Sendai virus C proteins, is fully capable of both counteracting the antiviral action of interferons and inhibiting viral RNA synthesis
Abstract
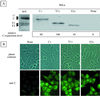
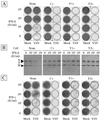
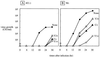
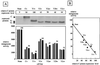
An open reading frame (ORF) overlapping the amino-terminal portion of the Sendai virus (SeV) P ORF in the +1 frame produces a nested set of carboxy-coterminal proteins, C', C, Y1, and Y2, which are referred to collectively as the C proteins. The C proteins are extremely versatile triple-role players; they counteract the antiviral action of interferons (IFNs), inhibit viral RNA synthesis, and are involved in virus assembly. In this study, we established HeLa cell lines stably expressing the C, Y1, and Y2 proteins individually and examined the capacities of these cells to circumvent the antiviral action of alpha/beta IFN (IFN-alpha/beta) and IFN-gamma and to inhibit viral transcription. The assay protocols included monitoring of IFN-alpha/beta-mediated signaling by interferon-stimulated response element-driven reporter gene expression and of the antiviral state induced by IFN-alpha/beta and IFN-gamma and measurement of reporter gene expression from an SeV minigenome, as well as quantification of SeV primary transcripts. When necessary, the activities measured were carefully normalized to the expression levels of the respective C proteins in cells. The data obtained clearly indicate that the smallest protein, Y2, was as active as the C and Y1 proteins in both counteracting the antiviral action of IFNs and inhibiting viral transcription. The data further show that intracellular transexpression of either C, Y1, or Y2 rendered HeLa cells moderately or only poorly permissive for not only wild-type SeV but also 4C(-) SeV, which expressed none of the four C proteins. On the basis of these findings, the roles of SeV C proteins in the natural life cycle are discussed.
Figures


Similar articles
-
Characterization of the amino acid residues of sendai virus C protein that are critically involved in its interferon antagonism and RNA synthesis down-regulation.J Virol. 2004 Jul;78(14):7443-54. doi: 10.1128/JVI.78.14.7443-7454.2004. J Virol. 2004. PMID: 15220418 Free PMC article.
-
The amino-terminal half of Sendai virus C protein is not responsible for either counteracting the antiviral action of interferons or down-regulating viral RNA synthesis.J Virol. 2002 Jul;76(14):7114-24. doi: 10.1128/jvi.76.14.7114-7124.2002. J Virol. 2002. PMID: 12072511 Free PMC article.
-
Versatility of the accessory C proteins of Sendai virus: contribution to virus assembly as an additional role.J Virol. 2000 Jun;74(12):5619-28. doi: 10.1128/jvi.74.12.5619-5628.2000. J Virol. 2000. PMID: 10823869 Free PMC article.
-
Paramyxovirus strategies for evading the interferon response.Rev Med Virol. 2002 Nov-Dec;12(6):337-57. doi: 10.1002/rmv.357. Rev Med Virol. 2002. PMID: 12410527 Review.
-
Paramyxovirus accessory proteins as interferon antagonists.Microbiol Immunol. 2001;45(12):787-800. doi: 10.1111/j.1348-0421.2001.tb01315.x. Microbiol Immunol. 2001. PMID: 11838896 Review.
Cited by
-
Mumps virus V protein antagonizes interferon without the complete degradation of STAT1.J Virol. 2005 Apr;79(7):4451-9. doi: 10.1128/JVI.79.7.4451-4459.2005. J Virol. 2005. PMID: 15767445 Free PMC article.
-
C-terminal region of STAT-1alpha is not necessary for its ubiquitination and degradation caused by mumps virus V protein.J Virol. 2002 Dec;76(24):12683-90. doi: 10.1128/jvi.76.24.12683-12690.2002. J Virol. 2002. PMID: 12438594 Free PMC article.
-
C Proteins: Controllers of Orderly Paramyxovirus Replication and of the Innate Immune Response.Viruses. 2022 Jan 12;14(1):137. doi: 10.3390/v14010137. Viruses. 2022. PMID: 35062341 Free PMC article. Review.
-
Rinderpest virus phosphoprotein gene is a major determinant of species-specific pathogenicity.J Virol. 2004 Jun;78(12):6676-81. doi: 10.1128/JVI.78.12.6676-6681.2004. J Virol. 2004. PMID: 15163758 Free PMC article.
-
An Insight into DNA-free Reprogramming Approaches to Generate Integration-free Induced Pluripotent Stem Cells for Prospective Biomedical Applications.Stem Cell Rev Rep. 2019 Apr;15(2):286-313. doi: 10.1007/s12015-018-9861-6. Stem Cell Rev Rep. 2019. PMID: 30417242 Review.
References
-
- Atreya P L, Kulkarni S. Respiratory syncytial virus A2 is resistant to the antiviral effects of type I interferons and human MxA. Virology. 1999;261:227–241. - PubMed
-
- Curran J, Marq J-B, Kolakofsky D. The Sendai virus nonstructural C proteins specifically inhibit viral mRNA synthesis. Virology. 1992;189:647–656. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

