Replication of naturally occurring woodchuck hepatitis virus deletion mutants in primary hepatocyte cultures and after transmission to naive woodchucks
- PMID: 11264370
- PMCID: PMC114872
- DOI: 10.1128/JVI.75.8.3811-3818.2001
Replication of naturally occurring woodchuck hepatitis virus deletion mutants in primary hepatocyte cultures and after transmission to naive woodchucks
Abstract
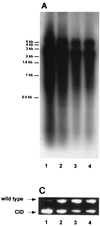
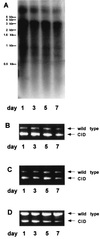
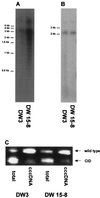
Woodchuck hepatitis virus (WHV) mutants with core internal deletions (CID) occur naturally in chronically WHV-infected woodchucks, as do hepatitis B virus mutants in humans. We studied the replication of WHV deletion mutants in primary woodchuck hepatocyte cultures and in vivo after transmission to naive woodchucks. By screening 14 wild-caught, chronically WHV-infected woodchucks, two woodchucks, WH69 and WH70, were found to harbor WHV CID mutants. Consistent with previous results, WHV CID mutants from both animals had deletions of variable lengths (90 to 135 bp) within the middle of the WHV core gene. In woodchuck WH69, WHV CID mutants represented a predominant fraction of the viral population in sera, normal liver tissues, and to a lesser extent, in liver tumor tissues. In primary hepatocytes of WH69, the replication of wild-type WHV and CID mutants was maintained at least for 7 days. Although WHV CID mutants were predominant in fractions of cellular WHV replicative intermediates, mutant covalently closed circular DNAs (cccDNAs) appeared to be a small part of cccDNA-enriched fractions. Analysis of cccDNA-enriched fractions from liver tissues of other woodchucks confirmed that mutant cccDNA represents only a small fraction of the total cccDNA pool. Four naive woodchucks were inoculated with sera from woodchuck WH69 or WH70 containing WHV CID mutants. All four woodchucks developed viremia after 3 to 4 weeks postinoculation (p.i.). They developed anti-WHV core antigen (WHcAg) antibody, lymphoproliferative response to WHcAg, and anti-WHV surface antigen. Only wild-type WHV, but no CID mutant, was found in sera from these woodchucks. The WHV CID mutant was also not identified in liver tissue from one woodchuck sacrificed in week 7 p.i. Three remaining woodchucks cleared WHV. Thus, the presence of WHV CID mutants in the inocula did not significantly change the course of acute self-limiting WHV infection. Our results indicate that the replication of WHV CID mutants might require some specific selective conditions. Further investigations on WHV CID mutants will allow us to have more insight into hepadnavirus replication.
Figures
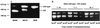



Similar articles
-
Naturally occurring woodchuck hepatitis virus (WHV) deletion mutants in chronically WHV-infected woodchucks.Virology. 2000 Nov 25;277(2):226-34. doi: 10.1006/viro.1999.0127. Virology. 2000. PMID: 11080471
-
Lack of WHV integration nearby N-myc2 and in the downstream b3n and win loci in a considerable fraction of liver tumors with activated N-myc2 from naturally infected wild woodchucks.Virology. 2006 Feb 5;345(1):258-69. doi: 10.1016/j.virol.2005.09.061. Epub 2005 Nov 3. Virology. 2006. PMID: 16271377
-
Kinetics of WHV-HDV replication in acute fatal course of woodchuck hepatitis.Arch Virol. 2008;153(11):2069-76. doi: 10.1007/s00705-008-0236-1. Epub 2008 Nov 6. Arch Virol. 2008. PMID: 18985276
-
Evaluation of new approaches to prophylactic and therapeutic vaccinations against hepatitis B viruses in the woodchuck model.Intervirology. 2001;44(2-3):124-31. doi: 10.1159/000050039. Intervirology. 2001. PMID: 11509873 Review.
-
The woodchuck model of hepatitis B virus infection.ILAR J. 2001;42(2):89-102. doi: 10.1093/ilar.42.2.89. ILAR J. 2001. PMID: 11406711 Review.
Cited by
-
Levels of hepatitis B virus replicative intermediate in serum samples of chronic hepatitis B patients.Mol Biol Rep. 2014 Jul;41(7):4689-96. doi: 10.1007/s11033-014-3339-7. Epub 2014 Apr 6. Mol Biol Rep. 2014. PMID: 24706057
-
Helper-dependent adenoviral vector-mediated delivery of woodchuck-specific genes for alpha interferon (IFN-alpha) and IFN-gamma: IFN-alpha but not IFN-gamma reduces woodchuck hepatitis virus replication in chronic infection in vivo.J Virol. 2004 Sep;78(18):10111-21. doi: 10.1128/JVI.78.18.10111-10121.2004. J Virol. 2004. PMID: 15331744 Free PMC article.
-
Aborted infection of human sodium taurocholate cotransporting polypeptide (hNTCP) expressing woodchuck hepatocytes with hepatitis B virus (HBV).Virus Genes. 2023 Dec;59(6):823-830. doi: 10.1007/s11262-023-02031-w. Epub 2023 Sep 20. Virus Genes. 2023. PMID: 37728707
-
In vivo transmission and dynamics of deleted genomes after experimental infection of woodchuck hepatitis B virus in adult animals.Virus Genes. 2002 Oct;25(2):147-57. doi: 10.1023/a:1020157717855. Virus Genes. 2002. PMID: 12416678
-
Woodchuck gamma interferon upregulates major histocompatibility complex class I transcription but is unable to deplete woodchuck hepatitis virus replication intermediates and RNAs in persistently infected woodchuck primary hepatocytes.J Virol. 2002 Jan;76(1):58-67. doi: 10.1128/jvi.76.1.58-67.2002. J Virol. 2002. PMID: 11739671 Free PMC article.
References
-
- Ackrill A M, Naoumov N V, Eddleston A L, Williams R. Specific deletions in the hepatitis B virus core open reading frame in patients with chronic active hepatitis B. J Med Virol. 1993;41:165–169. - PubMed
-
- Akarca U S, Lok A S. Naturally occurring core-gene-defective hepatitis B viruses. J Gen Virol. 1995;76:1821–1826. - PubMed
-
- Aye T T, Uchida T, Becker S O, Hirashima M, Shikata T, Komine F, Moriyama M, Arakawa Y, Mima S, Mizokami M, Lau J Y N. Variation of hepatitis B virus precore/core gene sequence in acute and fulminant hepatitis B. Dig Dis Sci. 1994;39:1281–1287. - PubMed
-
- Botta A, Lu M, Zheng X, Kemper T, Roggendorf M. Naturally occurring woodchuck hepatitis virus (WHV) deletion mutants in chronically WHV-infected woodchucks. Virology. 2000;277:226–234. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

