Herpes simplex virus type 1 promoter activity during latency establishment, maintenance, and reactivation in primary dorsal root neurons in vitro
- PMID: 11264377
- PMCID: PMC114879
- DOI: 10.1128/JVI.75.8.3885-3895.2001
Herpes simplex virus type 1 promoter activity during latency establishment, maintenance, and reactivation in primary dorsal root neurons in vitro
Abstract
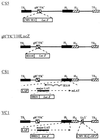
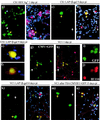
A neonatal rat dorsal root ganglion-derived neuronal culture system has been utilized to study herpes simplex virus (HSV) latency establishment, maintenance, and reactivation. We present our initial characterization of viral gene expression in neurons following infection with replication-defective HSV recombinants carrying beta-galactosidase and/or green fluorescent protein reporter genes under the control of lytic cycle- or latency-associated promoters. In this system lytic virus reporter promoter activity was detected in up to 58% of neurons 24 h after infection. Lytic cycle reporter promoters were shut down over time, and long-term survival of neurons harboring latent virus genomes was demonstrated. Latency-associated promoter-driven reporter gene expression was detected in neurons from early times postinfection and was stably maintained in up to 83% of neurons for at least 3 weeks. In latently infected cultures, silent lytic cycle promoters could be activated in up to 53% of neurons by nerve growth factor withdrawal or through inhibition of histone deacetylases by trichostatin A. We conclude that the use of recombinant viruses containing reporter genes, under the regulation of lytic and latency promoter control in neuronal cultures in which latency can be established and reactivation can be induced, is a potentially powerful system in which to study the molecular events that occur during HSV infection of neurons.
Figures

References
-
- Arthur J, Efstathiou S, Simmons A. Intranuclear foci containing low abundance herpes simplex virus latency-associated transcripts visualized by non-isotopic in situ hybridization. J Gen Virol. 1993;74:1363–1370. - PubMed
-
- Arthur J L, Everett R, Brierley I, Efstathiou S. Disruption of the 5′ and 3′ splice sites flanking the 2-kilobase latency-associated transcripts: evidence for alternate splicing in lytic and latent infections. J Gen Virol. 1998;79:107–116. - PubMed
-
- Babic N, Rodger G, Arthur J, Minson A C. A study of primary neuronal infection by mutants of herpes simplex virus type 1 lacking dispensable and non-dispensable glycoproteins. J Gen Virol. 1999;80:2403–2409. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

