Langat virus M protein is structurally homologous to prM
- PMID: 11264391
- PMCID: PMC114893
- DOI: 10.1128/JVI.75.8.3999-4001.2001
Langat virus M protein is structurally homologous to prM
Abstract
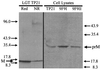
Langat (LGT) virus M protein has been generated in a recombinant system. Antiserum raised against the LGT virus M protein neutralizes tick-borne encephalitis serocomplex flaviviruses but not mosquito-borne flaviviruses, indicating that the M protein is exposed on the surface of virions. The antiserum recognizes intracellular LGT virus prM/M and binds to prM and M in Western blots of whole-cell lysates and purified virus, respectively. These data suggest that the prM and M proteins are structurally similar under native conditions and support the hypothesis that the "pr" portion of prM facilitates proper folding of the M protein for expression on the virion surface.
Figures

Similar articles
-
Amino acid substitution(s) in the stem-anchor region of langat virus envelope protein attenuates mouse neurovirulence.Virology. 2001 Jul 20;286(1):54-61. doi: 10.1006/viro.2001.0959. Virology. 2001. PMID: 11448158
-
Biophysical characterization and vector-specific antagonist activity of domain III of the tick-borne flavivirus envelope protein.J Virol. 2001 Apr;75(8):4002-7. doi: 10.1128/JVI.75.8.4002-4007.2001. J Virol. 2001. PMID: 11264392 Free PMC article.
-
Chimeric Langat/Dengue viruses protect mice from heterologous challenge with the highly virulent strains of tick-borne encephalitis virus.Virology. 2000 Aug 15;274(1):26-31. doi: 10.1006/viro.2000.0426. Virology. 2000. PMID: 10936085
-
Entry functions and antigenic structure of flavivirus envelope proteins.Novartis Found Symp. 2006;277:57-65; discussion 65-73, 251-3. Novartis Found Symp. 2006. PMID: 17319154 Review.
-
Analysis of flavivirus envelope proteins reveals variable domains that reflect their antigenicity and may determine their pathogenesis.Virus Res. 1995 Mar;35(3):307-21. doi: 10.1016/0168-1702(94)00090-y. Virus Res. 1995. PMID: 7785318 Review.
Cited by
-
Cytoarchitecture of ex vivo midgut cultures of unfed Ixodes scapularis infected with a tick-borne flavivirus.Ticks Tick Borne Dis. 2024 Mar;15(2):102301. doi: 10.1016/j.ttbdis.2023.102301. Epub 2023 Dec 21. Ticks Tick Borne Dis. 2024. PMID: 38134511 Free PMC article.
References
-
- Beaty B J, Calisher C H, Shope R E. Arboviruses. In: Lennette E H, Lennette E T, editors. Diagnostic procedures for viral, rickettsial and chlamydial infections. 7th ed. Washington, D.C.: American Public Health Association; 1995. pp. 189–212.
-
- Heinz F X, Stiasny K, Püschner-Auer G, Holzman H, Allison S L, Mandl C W, Kunz C. Structural changes and functional control of the tick-borne encephalitis virus glycoprotein E by the heterodimeric association with protein prM. Virology. 1994;198:109–117. - PubMed
-
- Kaufman B M, Summers P L, Dubois D R, Houston Cohen W, Gentry M K, Timchak R L, Burke D S, Eckels K H. Monoclonal antibodies for dengue virus prM glycoprotein protect mice against lethal dengue infection. Am J Trop Med Hyg. 1989;41:576–580. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

