The 3.7 A projection map of the glycerol facilitator GlpF: a variant of the aquaporin tetramer
- PMID: 11265760
- PMCID: PMC1084254
- DOI: 10.1093/embo-reports/kvd022
The 3.7 A projection map of the glycerol facilitator GlpF: a variant of the aquaporin tetramer
Abstract
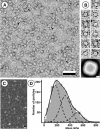
GlpF, the glycerol facilitator protein of Escherichia coli, is an archetypal member of the aquaporin superfamily. To assess its structure, recombinant histidine-tagged protein was overexpressed, solubilized in octylglucoside and purified to homogeneity. Negative stain electron microscopy of solubilized GlpF protein revealed a tetrameric structure of approximately 80 A side length. Scanning transmission electron microscopy yielded a mass of 170 kDa, corroborating the tetrameric nature of GlpF. Reconstitution of GlpF in the presence of lipids produced highly ordered two-dimensional crystals, which diffracted electrons to 3.6 A resolution. Cryoelectron microscopy provided a 3.7 A projection map exhibiting a unit cell comprised of two tetramers. In projection, GlpF is similar to AQP1, the erythrocyte water channel. However, the major density minimum within each monomer is distinctly larger in GlpF than in AQP1.
Figures



References
-
- Baumeister W., Karrenberg, F., Rachel, R., Engel, A., ten Heggeler, B. and Saxton, W.O. (1982) The major cell envelope protein of Micrococcus radiodurans (R1). Structural and chemical characterization. Eur. J. Biochem., 125, 535–544. - PubMed
-
- Bevington P.R. (1969) Data Reduction and Error Analysis for the Physical Sciences. McGraw–Hill, New York, NY.
-
- Borgnia M.J., Kozono, D., Calamita, G., Maloney, P.C. and Agre, P. (1999) Functional reconstitution and characterization of AqpZ, the E. coli water channel protein. J. Mol. Biol., 291, 1169–1179. - PubMed
-
- Bron P. et al. (1999) Oligomerization state of MIP proteins expressed in Xenopus oocytes as revealed by freeze-fracture electron-microscopy analysis. J. Struct. Biol., 128, 287–296. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

