ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis
- PMID: 11266364
- PMCID: PMC1083842
- DOI: 10.1093/embo-reports/kve046
ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis
Abstract
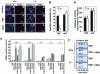
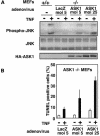
Apoptosis signal-regulating kinase (ASK) 1 is activated in response to various cytotoxic stresses including TNF, Fas and reactive oxygen species (ROS) such as H(2)O(2), and activates c-Jun NH(2)-terminal kinase (JNK) and p38. However, the roles of JNK and p38 signaling pathways during apoptosis have been controversial. Here we show that by deleting ASK1 in mice, TNF- and H(2)O(2)-induced sustained activations of JNK and p38 are lost in ASK1(-/-) embryonic fibroblasts, and that ASK1(-/-) cells are resistant to TNF- and H(2)O(2)-induced apoptosis. TNF- but not Fas-induced apoptosis requires ROS-dependent activation of ASK1-JNK/p38 pathways. Thus, ASK1 is selectively required for TNF- and oxidative stress-induced sustained activations of JNK/p38 and apoptosis.
Figures

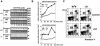

References
-
- Chang H., Nishitoh, H., Yang, X., Ichijo, H. and Baltimore D. (1998) Activation of apoptosis signal-regulating kinase 1 (ASK1) by the death adaptor Daxx. Science, 281, 1860–1863. - PubMed
-
- Chen Y.-R., Wang, X., Templeton, D., Davis, R. and Tan, T.-H. (1996) The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and γ-radiation. J. Biol. Chem., 50, 31929–31936. - PubMed
-
- Davis R.J. (2000) Signal transduction by the JNK group of MAP kinases. Cell, 103, 239–252. - PubMed
-
- Gross, A, McDonnell, J.M. and Korsmeyer, S.J. (1999) BCL-2 family members and the mitochondria in apoptosis. Genes Dev., 13, 1899–1911. - PubMed
-
- Guo Y.-L., Baysal, K., Kang, B., Yang, L.-J. and Williamson, J.R. (1998) Correlation between sustained c-Jun N-terminal protein kinase activation and apoptosis induced by tumor necrosis factor-α in rat mesangial cells. J. Biol. Chem., 273, 4027–4034. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

