Gradient of increasing affinity of importin beta for nucleoporins along the pathway of nuclear import
- PMID: 11266456
- PMCID: PMC2199621
- DOI: 10.1083/jcb.152.2.411
Gradient of increasing affinity of importin beta for nucleoporins along the pathway of nuclear import
Abstract
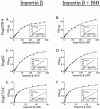
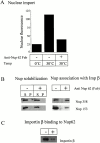
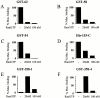
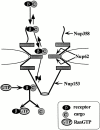
Nuclear import and export signals on macromolecules mediate directional, receptor-driven transport through the nuclear pore complex (NPC) by a process that is suggested to involve the sequential binding of transport complexes to different nucleoporins. The directionality of transport appears to be partly determined by the nucleocytoplasmic compartmentalization of components of the Ran GTPase system. We have analyzed whether the asymmetric localization of discrete nucleoporins can also contribute to transport directionality. To this end, we have used quantitative solid phase binding analysis to determine the affinity of an importin beta cargo complex for Nup358, the Nup62 complex, and Nup153, which are in the cytoplasmic, central, and nucleoplasmic regions of the NPC, respectively. These nucleoporins are proposed to provide progressively more distal binding sites for importin beta during import. Our results indicate that the importin beta transport complex binds to nucleoporins with progressively increasing affinity as the complex moves from Nup358 to the Nup62 complex and to Nup153. Antibody inhibition studies support the possibility that importin beta moves from Nup358 to Nup153 via the Nup62 complex during import. These results indicate that nucleoporins themselves, as well as the nucleocytoplasmic compartmentalization of the Ran system, are likely to play an important role in conferring directionality to nuclear protein import.
Figures
References
-
- Adam S.A., Sterne-Marr R., Gerace L. Nuclear protein import using digitonin-permeabilized cells. Methods Enzymol. 1992;219:97–110. - PubMed
-
- Berg O.G., Von Hippel P.H. Diffusion-controlled macromolecular interactions. Annu. Rev. Biophys. Biophys. Chem. 1985;14:131–160. - PubMed
-
- Bischoff F.R., Gorlich D. RanBP1 is crucial for the release of RanGTP from importin beta-related nuclear transport factors. FEBS Lett. 1997;419:249–254. - PubMed
-
- Chaillan-Huntington C., Braslavsky C.V., Kuhlmann J., Stewart M. Dissecting the interactions between NTF2, RanGDP, and the nucleoporin XFXFG repeats. J. Biol. Chem. 2000;275:5874–5879. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

