Drosophila aurora B kinase is required for histone H3 phosphorylation and condensin recruitment during chromosome condensation and to organize the central spindle during cytokinesis
- PMID: 11266459
- PMCID: PMC2195771
- DOI: 10.1083/jcb.152.4.669
Drosophila aurora B kinase is required for histone H3 phosphorylation and condensin recruitment during chromosome condensation and to organize the central spindle during cytokinesis
Abstract
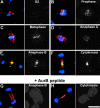
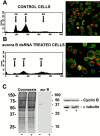
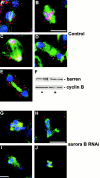
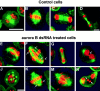
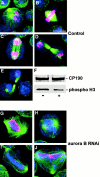
Aurora/Ipl1-related kinases are a conserved family of enzymes that have multiple functions during mitotic progression. Although it has been possible to use conventional genetic analysis to dissect the function of aurora, the founding family member in Drosophila (Glover, D.M., M.H. Leibowitz, D.A. McLean, and H. Parry. 1995. Cell. 81:95-105), the lack of mutations in a second aurora-like kinase gene, aurora B, precluded this approach. We now show that depleting Aurora B kinase using double-stranded RNA interference in cultured Drosophila cells results in polyploidy. aurora B encodes a passenger protein that associates first with condensing chromatin, concentrates at centromeres, and then relocates onto the central spindle at anaphase. Cells depleted of the Aurora B kinase show only partial chromosome condensation at mitosis. This is associated with a reduction in levels of the serine 10 phosphorylated form of histone H3 and a failure to recruit the Barren condensin protein onto chromosomes. These defects are associated with abnormal segregation resulting from lagging chromatids and extensive chromatin bridging at anaphase, similar to the phenotype of barren mutants (Bhat, M.A., A.V. Philp, D.M. Glover, and H.J. Bellen. 1996. Cell. 87:1103-1114.). The majority of treated cells also fail to undertake cytokinesis and show a reduced density of microtubules in the central region of the spindle. This is accompanied by a failure to correctly localize the Pavarotti kinesin-like protein, essential for this process. We discuss these conserved functions of Aurora B kinase in chromosome transmission and cytokinesis.
Figures


References
-
- Adams R.R., Wheatleya S.P., Gouldsworthy A.M., Kandels-Lewis S.E., Carmena M., Smythe C., Gerloff D.L., Earnshaw W.C. INCENP binds the aurora-related kinase AIRK2 and is required to target it to chromosomes, the central spindle and cleavage furrow. Curr. Biol. 2000;10:1075–1078. - PubMed
-
- Bhat M.A., Philp A.V., Glover D.M., Bellen H.J. Chromatid segregation at anaphase requires the barren product, a novel chromosome-associated protein that interacts with Topoisomerase II. Cell. 1996;87:1103–1114. - PubMed
-
- Bradbury E.M. Reversible histone modifications and the chromosome cell cycle. Bioessays. 1992;14:9–16. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

