The plasminogen activator inhibitor PAI-1 controls in vivo tumor vascularization by interaction with proteases, not vitronectin. Implications for antiangiogenic strategies
- PMID: 11266468
- PMCID: PMC2195770
- DOI: 10.1083/jcb.152.4.777
The plasminogen activator inhibitor PAI-1 controls in vivo tumor vascularization by interaction with proteases, not vitronectin. Implications for antiangiogenic strategies
Abstract
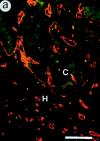
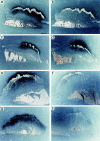
The plasminogen (Plg)/plasminogen activator (PA) system plays a key role in cancer progression, presumably via mediating extracellular matrix degradation and tumor cell migration. Consequently, urokinase-type PA (uPA)/plasmin antagonists are currently being developed for suppression of tumor growth and angiogenesis. Paradoxically, however, high levels of PA inhibitor 1 (PAI-1) are predictive of a poor prognosis for survival of patients with cancer. We demonstrated previously that PAI-1 promoted tumor angiogenesis, but by an unresolved mechanism. We anticipated that PAI-1 facilitated endothelial cell migration via its known interaction with vitronectin (VN) and integrins. However, using adenoviral gene transfer of PAI-1 mutants, we observed that PAI-1 promoted tumor angiogenesis, not by interacting with VN, but rather by inhibiting proteolytic activity, suggesting that excessive plasmin proteolysis prevents assembly of tumor vessels. Single deficiency of uPA, tissue-type PA (tPA), uPA receptor, or VN, as well as combined deficiencies of uPA and tPA did not impair tumor angiogenesis, whereas lack of Plg reduced it. Overall, these data indicate that plasmin proteolysis, even though essential, must be tightly controlled during tumor angiogenesis, probably to allow vessel stabilization and maturation. These data provide insights into the clinical paradox whereby PAI-1 promotes tumor progression and warrant against the uncontrolled use of uPA/plasmin antagonists as tumor angiogenesis inhibitors.
Figures














References
-
- Andreasen P.A., Kjoller L., Christensen L., Duffy M.J. The urokinase-type plasminogen activator system in cancer metastasisa review. Int. J. Cancer. 1997;72:1–22. - PubMed
-
- Bajou K., Noël A., Gerard R.D., Masson V., Brunner N., Holst-Hansen C., Skobe M., Fusenig N.E., Carmeliet P., Collen D., Foidart J.M. Absence of host plasminogen activator inhibitor 1 prevents cancer invasion and vascularization. Nat. Med. 1998;4:923–928. - PubMed
-
- Blasi F. uPA, uPAR, PAI-1key intersection of proteolytic, adhesive and chemotactic highways? Immunol. Today. 1997;18:415–417. - PubMed
-
- Brunner N., Stephens R.W., Dano K. Control of invasion and metastasis. In: Harris J.R., editor. Disease of the Breast. Lippincott; Williams & Wilkins, Philadelphia, PA: 2000. pp. 367–375.
-
- Bugge T.H., Flick M.J., Daugherty C.C., Degen J.L. Plasminogen deficiency causes severe thrombosis but is compatible with development and reproduction. Genes Dev. 1995;9:794–807. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

