Rab27a regulates the peripheral distribution of melanosomes in melanocytes
- PMID: 11266470
- PMCID: PMC2195786
- DOI: 10.1083/jcb.152.4.795
Rab27a regulates the peripheral distribution of melanosomes in melanocytes
Abstract
Rab GTPases are regulators of intracellular membrane traffic. We report a possible function of Rab27a, a protein implicated in several diseases, including Griscelli syndrome, choroideremia, and the Hermansky-Pudlak syndrome mouse model, gunmetal. We studied endogenous Rab27a and overexpressed enhanced GFP-Rab27a fusion protein in several cultured melanocyte and melanoma-derived cell lines. In pigmented cells, we observed that Rab27a decorates melanosomes, whereas in nonpigmented cells Rab27a colocalizes with melanosome-resident proteins. When dominant interfering Rab27a mutants were expressed in pigmented cells, we observed a redistribution of pigment granules with perinuclear clustering. This phenotype is similar to that observed by others in melanocytes derived from the ashen and dilute mutant mice, which bear mutations in the Rab27a and MyoVa loci, respectively. We also found that myosinVa coimmunoprecipitates with Rab27a in extracts from melanocytes and that both Rab27a and myosinVa colocalize on the cytoplasmic face of peripheral melanosomes in wild-type melanocytes. However, the amount of myosinVa in melanosomes from Rab27a-deficient ashen melanocytes is greatly reduced. These results, together with recent data implicating myosinVa in the peripheral capture of melanosomes, suggest that Rab27a is necessary for the recruitment of myosinVa, so allowing the peripheral retention of melanosomes in melanocytes.
Figures
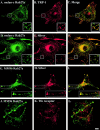


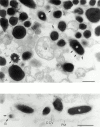

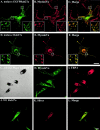
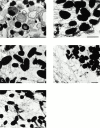

Comment in
-
Of yeast, mice, and men. Rab proteins and organelle transport.J Cell Biol. 2001 Feb 19;152(4):F21-4. doi: 10.1083/jcb.152.4.f21. J Cell Biol. 2001. PMID: 11266477 Free PMC article. No abstract available.
References
-
- Allan B.B., Moyer B.D., Balch W.E. Rab1 recruitment of p115 into a cis-SNARE complexprogramming budding COPII vesicles for fusion. Science. 2000;289:444–448. - PubMed
-
- Bennett D.C., Cooper P.J., Hart I.R. A line of non-tumorigenic mouse melanocytes, syngeneic with the B16 melanoma and requiring a tumour promoter for growth. Int. J. Cancer. 1987;39:414–418. - PubMed
-
- Bennett D.C., Cooper P.J., Dexter T.J., Devlin L.M., Heasman J., Nester B. Cloned mouse melanocyte lines carrying the germline mutations albino and browncomplementation in culture. Development. 1989;105:379–385. - PubMed
-
- Calvo P.A., Frank D.W., Bieler B.M., Berson J.F., Marks M.S. A cytoplasmic sequence in human tyrosinase defines a second class of di-leucine-based sorting signals for late endosomal and lysosomal delivery. J. Biol. Chem. 1999;274:12780–12789. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

