Distinct protein sorting and localization to premelanosomes, melanosomes, and lysosomes in pigmented melanocytic cells
- PMID: 11266471
- PMCID: PMC2195785
- DOI: 10.1083/jcb.152.4.809
Distinct protein sorting and localization to premelanosomes, melanosomes, and lysosomes in pigmented melanocytic cells
Abstract
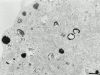
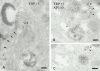
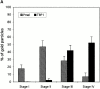
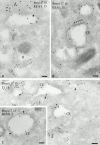
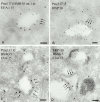
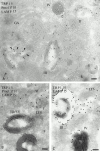
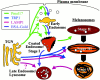
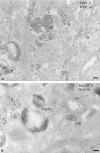
Melanosomes and premelanosomes are lysosome-related organelles with a unique structure and cohort of resident proteins. We have positioned these organelles relative to endosomes and lysosomes in pigmented melanoma cells and melanocytes. Melanosome resident proteins Pmel17 and TRP1 localized to separate vesicular structures that were distinct from those enriched in lysosomal proteins. In immunogold-labeled ultrathin cryosections, Pmel17 was most enriched along the intralumenal striations of premelanosomes. Increased pigmentation was accompanied by a decrease in Pmel17 and by an increase in TRP1 in the limiting membrane. Both proteins were largely excluded from lysosomal compartments enriched in LAMP1 and cathepsin D. By kinetic analysis of fluid phase uptake and immunogold labeling, premelanosomal proteins segregated from endocytic markers within an unusual endosomal compartment. This compartment contained Pmel17, was accessed by BSA-gold after 15 min, was acidic, and displayed a cytoplasmic planar coat that contained clathrin. Our results indicate that premelanosomes and melanosomes represent a distinct lineage of organelles, separable from conventional endosomes and lysosomes within pigmented cells. Furthermore, they implicate an unusual clathrin-coated endosomal compartment as a site from which proteins destined for premelanosomes and lysosomes are sorted.
Figures



Comment in
-
Of yeast, mice, and men. Rab proteins and organelle transport.J Cell Biol. 2001 Feb 19;152(4):F21-4. doi: 10.1083/jcb.152.4.f21. J Cell Biol. 2001. PMID: 11266477 Free PMC article. No abstract available.
References
-
- Bhatnagar V., Anjaiah S., Puri N., Darshanam N.A., Ramaiah A. pH of melanosomes of B16 murine melanoma is acidicits physiological importance in the regulation of melanin biosynthesis. Arch. Biochem. Biophys. 1993;307:183–192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

