Rab27a: A key to melanosome transport in human melanocytes
- PMID: 11266474
- PMCID: PMC2195788
- DOI: 10.1083/jcb.152.4.843
Rab27a: A key to melanosome transport in human melanocytes
Abstract
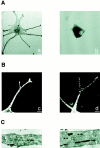
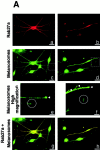
Normal pigmentation depends on the uniform distribution of melanin-containing vesicles, the melanosomes, in the epidermis. Griscelli syndrome (GS) is a rare autosomal recessive disease, characterized by an immune deficiency and a partial albinism that has been ascribed to an abnormal melanosome distribution. GS maps to 15q21 and was first associated with mutations in the myosin-V gene. However, it was demonstrated recently that GS can also be caused by a mutation in the Rab27a gene. These observations prompted us to investigate the role of Rab27a in melanosome transport. Using immunofluorescence and immunoelectron microscopy studies, we show that in normal melanocytes Rab27a colocalizes with melanosomes. In melanocytes isolated from a patient with GS, we show an abnormal melanosome distribution and a lack of Rab27a expression. Finally, reexpression of Rab27a in GS melanocytes restored melanosome transport to dendrite tips, leading to a phenotypic reversion of the diseased cells. These results identify Rab27a as a key component of vesicle transport machinery in melanocytes.
Figures





Comment in
-
Of yeast, mice, and men. Rab proteins and organelle transport.J Cell Biol. 2001 Feb 19;152(4):F21-4. doi: 10.1083/jcb.152.4.f21. J Cell Biol. 2001. PMID: 11266477 Free PMC article. No abstract available.
References
-
- Aberdam E., Romero C., Ortonne J.P. Repeated UVB irradiations do not have the same potential to promote stimulation of melanogenesis in cultured normal human melanocytes. J. Cell Sci. 1993;106:1015–1022. - PubMed
-
- Buscà R., Martinez M., Vilella E., Pognonec P., Deeb S., Auwerw J., Reina M., Vilaro S. The mutation Gly142-->Glu in human lipoprotein lipase produces a missorted protein that is diverted to lysosomes. J. Biol. Chem. 1996;271:2139–2146. - PubMed
-
- Cheney R.E., O'Shea M.K., Heuser J.E., Coelho M.V., Wolenski J.S., Espreafico E.M., Forscher P., Larson R.E., Mooseker M.S. Brain myosin-V is a two-headed unconventional myosin with motor activity. Cell. 1993;75:13–23. - PubMed
-
- Dufourcq-Lagelouse R., Pastural E., Barrat F.J., Feldmann J., Le Deist F., Fischer A., De Saint Basile G. Genetic basis of hemophagocytic lymphohistiocytosis syndrome. Int. J. Mol. Med. 1999;4:127–133. - PubMed
-
- Echard A., Jollivet F., Martinez O., Lacapere J.J., Rousselet A., Janoueix-Lerosey I., Goud B. Interaction of a Golgi-associated kinesin-like protein with Rab6. Science. 1998;279:580–585. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

