Cooperative binding of effectors by an allosteric ribozyme
- PMID: 11266567
- PMCID: PMC31269
- DOI: 10.1093/nar/29.7.1631
Cooperative binding of effectors by an allosteric ribozyme
Abstract
An allosteric ribozyme that requires two different effectors to induce catalysis was created using modular rational design. This ribozyme construct comprises five conjoined RNA modules that operate in concert as an obligate FMN- and theophylline-dependent molecular switch. When both effectors are present, this 'binary' RNA switch self-cleaves with a rate enhancement of approximately 300-fold over the rate observed in the absence of effectors. Kinetic and structural studies implicate a switching mechanism wherein FMN binding induces formation of the active ribozyme conformation. However, the binding site for FMN is rendered inactive unless theophylline first binds to its corresponding site and reorganizes the RNA structure. This example of cooperative binding between allosteric effectors reveals a level of structural and functional complexity for RNA that is similar to that observed with allosteric proteins.
Figures

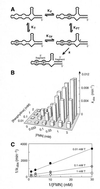

References
-
- Tang J. and Breaker,R.R. (1997) Rational design of allosteric ribozymes. Chem. Biol., 4, 453–459. - PubMed
-
- Soukup G.A. and Breaker,R.R. (1999) Design of allosteric hammerhead ribozymes activated by ligand-induced structure stabilization. Structure, 7, 783–791. - PubMed
-
- Breaker R.R. (1997) In vitro selection of catalytic polynucleotides. Chem. Rev., 97, 371–390. - PubMed
-
- Li Y. and Breaker,R.R. (1999) Deoxyribozymes: new players in the ancient game of biocatalysis. Curr. Opin. Struct. Biol., 9, 315–323. - PubMed
-
- Wilson D.S. and Szostak,J.W. (1999) In vitro selection of functional nucleic acids. Annu. Rev. Biochem., 68, 611–647. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

