The effect of substrate binding on the conformation and structural stability of Herpes simplex virus type 1 thymidine kinase
- PMID: 11266595
- PMCID: PMC2249856
- DOI: 10.1110/ps.27401
The effect of substrate binding on the conformation and structural stability of Herpes simplex virus type 1 thymidine kinase
Abstract
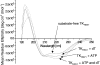
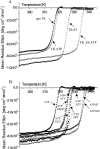
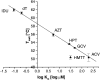
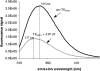
The structure of Herpes simplex virus type 1 thymidine kinase (TK(HSV1)) is known at high resolution in complex with a series of ligands and exhibits important structural similarities to the nucleoside monophosphate (NMP) kinase family, which are known to show large conformational changes upon binding of substrates. The effect of substrate binding on the conformation and structural stability of TK(HSV1), measured by thermal denaturation experiments, far-UV circular dichroism (CD) and fluorescence is described, and the results indicate that the conformation of the ligand-free TK(HSV1) is less ordered and less stable compared to the ligated enzyme. Furthermore, two crystal structures of TK(HSV1) in complex with two new ligands, HPT and HMTT, refined to 2.2 A are presented. Although TK(HSV1):HPT does not exhibit any significant deviations from the model of TK(HSV1):dT, the TK(HSV1):HMTT complex displays a unique conformationally altered active site resulting in a lowered thermal stability of this complex. Moreover, we show that binding affinity and binding mode of the ligand correlate with thermal stability of the complex. We use this correlation to propose a method to estimate binding constants for new TK(HSV1)substrates using thermal denaturation measurements monitored by CD spectroscopy. The kinetic and structural results of both test substrates HPT and HMTT show that the CD thermal denaturation system is very sensitive to conformational changes caused by unusual binding of a substrate analog.
Figures

Similar articles
-
Folding and self-assembly of herpes simplex virus type 1 thymidine kinase.J Mol Biol. 2001 Oct 26;313(3):657-70. doi: 10.1006/jmbi.2001.5060. J Mol Biol. 2001. PMID: 11676546
-
Kinetics and crystal structure of the wild-type and the engineered Y101F mutant of Herpes simplex virus type 1 thymidine kinase interacting with (North)-methanocarba-thymidine.Biochemistry. 2000 Aug 8;39(31):9597-603. doi: 10.1021/bi000668q. Biochemistry. 2000. PMID: 10924157
-
Integrated homology modelling and X-ray study of herpes simplex virus I thymidine kinase: a case study.J Recept Signal Transduct Res. 1997 Jan-May;17(1-3):475-94. doi: 10.3109/10799899709036622. J Recept Signal Transduct Res. 1997. PMID: 9029509 Review.
-
Biochemical and structural characterization of (South)-methanocarbathymidine that specifically inhibits growth of herpes simplex virus type 1 thymidine kinase-transduced osteosarcoma cells.J Biol Chem. 2004 Jul 30;279(31):32832-8. doi: 10.1074/jbc.M313343200. Epub 2004 May 25. J Biol Chem. 2004. PMID: 15163659
-
Herpes simplex virus thymidine kinase as a marker/reporter gene for PET imaging of gene therapy.Q J Nucl Med. 1999 Jun;43(2):163-9. Q J Nucl Med. 1999. PMID: 10429512 Review.
Cited by
-
Discovery of first-in-class nanomolar inhibitors of heptosyltransferase I reveals a new aminoglycoside target and potential alternative mechanism of action.Sci Rep. 2022 May 4;12(1):7302. doi: 10.1038/s41598-022-10776-x. Sci Rep. 2022. PMID: 35508636 Free PMC article.
-
Testing the sensitivities of noncognate inhibitors to varicella zoster virus thymidine kinase: implications for postherpetic neuralgia therapy with existing agents.J Mol Model. 2014 Jul;20(7):2321. doi: 10.1007/s00894-014-2321-6. Epub 2014 Jun 25. J Mol Model. 2014. PMID: 24961898
-
Crystal structure of poxvirus thymidylate kinase: an unexpected dimerization has implications for antiviral therapy.Proc Natl Acad Sci U S A. 2008 Nov 4;105(44):16900-5. doi: 10.1073/pnas.0804525105. Epub 2008 Oct 29. Proc Natl Acad Sci U S A. 2008. PMID: 18971333 Free PMC article.
References
-
- Balzarini, J., De Clercq, E., Baumgartner, H., Bodenteich, M., and Griengl, H. 1989. Carbocyclic 5-Iodo-2′-deoxyuridine (C-IDU) and carbocyclic (E)-5–(2-Bromovinyl)-2′-deoxyuridine (C-BVDU) as unique example of chiral molecules where the two enantiomeric forms are biologically active: interaction of the (+)- and (−)-enantiomers of C-IDU and C-BVDU with the thymidine kinase if herpes simplex virus type 1. Mol. Pharmacol. 37 395–401. - PubMed
-
- Bennett, M.S., Wien, F., Champness, J.N., Batuwangala, T., Rutherford, T., Summers, W.C., Sun, H., Wright, G., and Sanderson, M.R. 1999. Structure to 1.9 A resolution of a complex with herpes simplex virus type-1 thymidine kinase of a novel, non-substrate inhibitor: X-ray crystallographic comparison with binding of aciclovir. FEBS Lett. 443 121–125. - PubMed
-
- Bonini, C., Ferrari, G., Verzeletti, S., Servida, P., Zappone, E., Ruggieri, L., Ponzoni, M., Rossini, S., Mavilio, F., Traversari, C., and Bordignon, C. 1997. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia [see comments]. Science 276 1719–1724. - PubMed
-
- Bradford, M.M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochem. 72 248–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

