Ulex europaeus agglutinin II (UEA-II) is a novel, potent inhibitor of complement activation
- PMID: 11266613
- PMCID: PMC2373946
- DOI: 10.1110/ps.26401
Ulex europaeus agglutinin II (UEA-II) is a novel, potent inhibitor of complement activation
Abstract
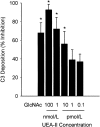
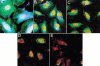
Complement is an important mediator of vascular injury following oxidative stress. We recently demonstrated that complement activation following endothelial oxidative stress is mediated by mannose-binding lectin (MBL) and activation of the lectin complement pathway. Here, we investigated whether nine plant lectins which have a binding profile similar to that of MBL competitively inhibit MBL deposition and subsequent complement activation following human umbilical vein endothelial cell (HUVEC) oxidative stress. HUVEC oxidative stress (1% O(2), 24 hr) significantly increased Ulex europaeus agglutinin II (UEA-II) binding by 72 +/- 9% compared to normoxic cells. UEA-II inhibited MBL binding to HUVEC in a concentration-dependent manner following oxidative stress. Further, MBL inhibited UEA-II binding to HUVEC in a concentration-dependent manner following oxidative stress, suggesting a common ligand. UEA-II (< or = 100 micromol/L) did not attenuate the hemolytic activity, nor did it inhibit C3a des Arg formation from alternative or classical complement pathway-specific hemolytic assays. C3 deposition (measured by ELISA) following HUVEC oxidative stress was inhibited by UEA-II in a concentration-dependent manner (IC(50) = 10 pmol/L). UEA-II inhibited C3 and MBL co-localization (confocal microscopy) in a concentration-dependent manner on HUVEC following oxidative stress (IC(50) approximately 1 pmol/L). Finally, UEA-II significantly inhibited complement-dependent neutrophil chemotaxis, but failed to inhibit fMLP-mediated chemotaxis, following endothelial oxidative stress. These data demonstrate that UEA-II is a novel, potent inhibitor of human MBL deposition and complement activation following human endothelial oxidative stress.
Figures




References
-
- Bless, N.M., Warner, R.L., Padgaonkar, V.A., Lentsch, A.B., Czermak, B.J., Schmal, H., Friedl, H.P., and Ward, P.A. 1999. Roles for C-X-C chemokines and C5a in lung injury after hindlimb ischemia-reperfusion. Am J Physiol 276 L57–L63. - PubMed
-
- Buerke, M., Prüfer, D., Dahm, M., Oelert, H., Meyer, J., and Darius, H. 1998. Blocking of classical complement pathway inhibits endothelial adhesion molecule expression and preserves ischemic myocardium from reperfusion injury. Journal of Pharmacology and Experimental Therapeutics 286 429–438. - PubMed
-
- Collard, C.D., Agah, A., Reenstra, W.R., Buras, J., and Stahl, G.L. 1999. Endothelial nuclear factor-kB translocation and vascular cell adhesion molecule-1 induction by complement: Inhibition with anti-human C5 therapy or cGMP analogues. Arterioscler Thromb Vasc Biol 19 2623–2629. - PubMed
-
- Collard, C.D., Vakeva, A., Bukusoglu, C., Zund, G., Sperati, C.J., Colgan, S.P., and Stahl, G.L. 1997. Reoxygenation of hypoxic human umbilical vein endothelial cells (HUVECs) activates the classic complement pathway. Circulation 96 326–333. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

