Solvation energetics and conformational change in EF-hand proteins
- PMID: 11266616
- PMCID: PMC2373930
- DOI: 10.1110/ps.33601
Solvation energetics and conformational change in EF-hand proteins
Abstract
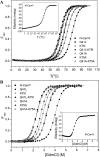
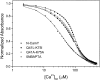
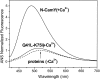
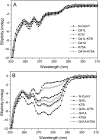
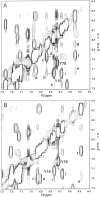
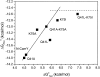
Calmodulin and other members of the EF-hand protein family are known to undergo major changes in conformation upon binding Ca(2+). However, some EF-hand proteins, such as calbindin D9k, bind Ca(2+) without a significant change in conformation. Here, we show the importance of a precise balance of solvation energetics to conformational change, using mutational analysis of partially buried polar groups in the N-terminal domain of calmodulin (N-cam). Several variants were characterized using fluorescence, circular dichroism, and NMR spectroscopy. Strikingly, the replacement of polar side chains glutamine and lysine at positions 41 and 75 with nonpolar side chains leads to dramatic enhancement of the stability of the Ca(2+)-free state, a corresponding decrease in Ca(2+)-binding affinity, and an apparent loss of ability to change conformation to the open form. The results suggest a paradigm for conformational change in which energetic strain is accumulated in one state in order to modulate the energetics of change to the alternative state.
Figures


References
-
- Ackers, G.K., Doyle, M.L., Myers, D., and Daugherty, M.A. 1992. Molecular code for cooperativity in hemoglobin. Science 255 54–63. - PubMed
-
- Babu, Y.S., Bugg, C.E., and Cook, W.J. 1988. Structure of calmodulin refined at 2.2 Å resolution. J. Mol. Biol. 204 191–204. - PubMed
-
- Bayley, P., Ahlstrom, P., Martin, S.R., and Forsen, S. 1984. The kinetics of calcium binding to calmodulin: Quin 2 and ANS stopped-flow fluorescence studies. Biochem. Biophys. Res. Commun. 120: 185–91. - PubMed
-
- Biekofsky, R.R., Martin, S.R., Browne, J.P., Bayley, P.M., and Feeney, J. 1998. Ca2+ coordination to backbone carbonyl oxygen atoms in calmodulin and other EF-hand proteins: 15N chemical shifts as probes for monitoring individual-site Ca2+ coordination. Biochemistry 37 7617–29. - PubMed
-
- Blaber, M., Lindstrom, J.D., Gassner, N., Xu, J., Heinz, D.W., and Matthews, B.W. 1993. Energetic cost and structural consequences of burying a hydroxyl group within the core of a protein determined from Ala–Ser and Val–Thr substitutions in T4 lysozyme. Biochemistry 32 11363–11373. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

