Stabilization of hen egg white lysozyme by a cavity-filling mutation
- PMID: 11266617
- PMCID: PMC2373952
- DOI: 10.1110/ps.37401
Stabilization of hen egg white lysozyme by a cavity-filling mutation
Abstract
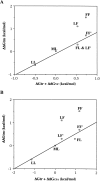
Stabilization of a protein using cavity-filling strategy has hardly been successful because of unfavorable van der Waals contacts. We succeeded in stabilizing lysozymes by cavity-filling mutations. The mutations were checked by a simple energy minimization in advance. It was shown clearly that the sum of free energy change caused by the hydrophobicity and the cavity size was correlated very well with protein stability. We also considered the aromatic-aromatic interaction. It is reconfirmed that the cavity-filling mutation in a hydrophobic core is a very useful method to stabilize a protein when the mutation candidate is selected carefully.
Figures

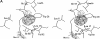
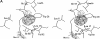

Similar articles
-
A mutational analysis of binding interactions in an antigen-antibody protein-protein complex.Biochemistry. 1998 Jun 2;37(22):7981-91. doi: 10.1021/bi980148j. Biochemistry. 1998. PMID: 9609690
-
Cooperative folding of the isolated alpha-helical domain of hen egg-white lysozyme.J Mol Biol. 2001 Nov 23;314(2):321-9. doi: 10.1006/jmbi.2001.5122. J Mol Biol. 2001. PMID: 11718563
-
Crystal structures of K33 mutant hen lysozymes with enhanced activities.J Biochem. 2008 Nov;144(5):619-23. doi: 10.1093/jb/mvn108. Epub 2008 Sep 6. J Biochem. 2008. PMID: 18776207
-
Engineering of lysozyme.EXS. 1996;75:163-81. doi: 10.1007/978-3-0348-9225-4_10. EXS. 1996. PMID: 8765300 Review.
-
Fluctuations in protein structure from X-ray diffraction.Annu Rev Biophys Bioeng. 1984;13:331-71. doi: 10.1146/annurev.bb.13.060184.001555. Annu Rev Biophys Bioeng. 1984. PMID: 6331286 Review. No abstract available.
Cited by
-
Cavities of alpha(1)-antitrypsin that play structural and functional roles.Protein Sci. 2001 Jul;10(7):1446-53. doi: 10.1110/ps.840101. Protein Sci. 2001. PMID: 11420446 Free PMC article.
-
Energetics of aliphatic deletions in protein cores.Protein Sci. 2006 Aug;15(8):1858-72. doi: 10.1110/ps.062274906. Protein Sci. 2006. PMID: 16877708 Free PMC article.
-
Mutagenic stabilization and/or disruption of a CD4-bound state reveals distinct conformations of the human immunodeficiency virus type 1 gp120 envelope glycoprotein.J Virol. 2002 Oct;76(19):9888-99. doi: 10.1128/jvi.76.19.9888-9899.2002. J Virol. 2002. PMID: 12208966 Free PMC article.
-
Protein Engineering of the Soluble Metal-dependent Formate Dehydrogenase from Escherichia coli.Anal Sci. 2021 May 10;37(5):733-739. doi: 10.2116/analsci.20SCP15. Epub 2021 Jan 15. Anal Sci. 2021. PMID: 33455969
-
Comparing Residue Clusters from Thermophilic and Mesophilic Enzymes Reveals Adaptive Mechanisms.PLoS One. 2016 Jan 7;11(1):e0145848. doi: 10.1371/journal.pone.0145848. eCollection 2016. PLoS One. 2016. PMID: 26741367 Free PMC article.
References
-
- Akasako, A., Haruki, M., Oobatake, M., and Kanaya, S. 1997. Conformational stabilities of Escherichia coli RNase HI variants with a series of amino acid substitutions at a cavity within the hydrophobic core. J. Biol. Chem. 272 18686–18693. - PubMed
-
- Baldwin, E., Xu, J., Hajiseyedjavadi, O., Baase, W.A., and Mattews, B.W. 1996. Thermodynamic and structural compensation in "size-switch" core repacking variants of bacteriophage T4 lysozyme. J. Mol. Biol. 259 542–559. - PubMed
-
- Brunger, A.T. 1992. X-PLOR manual Ver. 3.1. Yale University, NH, USA.
-
- Buckle, A.M., Cramer, P., and Fersht, A.R. 1996. Structural and energetic responses to cavity-creating mutations in hydrophobic cores: Observation of a buried water molecule and the hydrophilic nature of such hydrophobic cavities. Biochemistry 35 4298–4305. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

