Enantioselectivity in Candida antarctica lipase B: a molecular dynamics study
- PMID: 11266619
- PMCID: PMC2373953
- DOI: 10.1110/ps.33901
Enantioselectivity in Candida antarctica lipase B: a molecular dynamics study
Abstract
A major problem in predicting the enantioselectivity of an enzyme toward substrate molecules is that even high selectivity toward one substrate enantiomer over the other corresponds to a very small difference in free energy. However, total free energies in enzyme-substrate systems are very large and fluctuate significantly because of general protein motion. Candida antarctica lipase B (CALB), a serine hydrolase, displays enantioselectivity toward secondary alcohols. Here, we present a modeling study where the aim has been to develop a molecular dynamics-based methodology for the prediction of enantioselectivity in CALB. The substrates modeled (seven in total) were 3-methyl-2-butanol with various aliphatic carboxylic acids and also 2-butanol, as well as 3,3-dimethyl-2-butanol with octanoic acid. The tetrahedral reaction intermediate was used as a model of the transition state. Investigative analyses were performed on ensembles of nonminimized structures and focused on the potential energies of a number of subsets within the modeled systems to determine which specific regions are important for the prediction of enantioselectivity. One category of subset was based on atoms that make up the core structural elements of the transition state. We considered that a more favorable energetic conformation of such a subset should relate to a greater likelihood for catalysis to occur, thus reflecting higher selectivity. The results of this study conveyed that the use of this type of subset was viable for the analysis of structural ensembles and yielded good predictions of enantioselectivity.
Figures




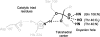

References
-
- Berglund, P., Vallikivi, I., Fransson, L., Dannacher, H., Holmquist, M., Martinelle, M., Björkling, F., Parve, O., and Hult, K. 1999. Switched enantiopreference of Humicola lipase for 2-phenoxyalkanoic acid ester homologs can be rationalized by different substrate binding modes. Tetrahedron: Asymmetry 10 4191–4202.
-
- Berthod, H. and Pullman, A. 1965. Sur le calcul des caractéristiques du squelette σ des molécules conjuguées. J. Chim. Phys. 62 942–946.
-
- Berthod, H., Giessner-Prettre, C.L., and Pullman, A. 1967. Sur les rÔles respectifs des électrons σ et π dans les propriétés des dérivés halogénés des molécules conjuguées. Application Á l'étude de l'uracile et du fluorouracile. Theor. Chim. Acta. 8 212–222.
-
- Chen, C.S., Fujimoto, Y., Girdaukas, G., and Sih, C.J. 1982. Quantitative analysis of biochemical kinetic resolutions of enantiomers. J. Am. Chem. Soc. 104 7294–7299.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources

