An SH2-domain-containing kinase negatively regulates the phosphatidylinositol-3 kinase pathway
- PMID: 11274054
- PMCID: PMC312652
- DOI: 10.1101/gad.871001
An SH2-domain-containing kinase negatively regulates the phosphatidylinositol-3 kinase pathway
Abstract
SHK1 is a novel dual-specificity kinase that contains an SH2 domain in its C-terminal region. We demonstrate that SHK1 is required for proper chemotaxis and phagocytosis. Mutant shk1 null cells lack polarity, move very slowly, and exhibit an elevated and temporally extended chemoattractant-mediated activation of the kinase Akt/PKB. GFP fusions of the PH domain of Akt/PKB or the PH-domain-containing protein CRAC, which become transiently associated with the plasma membrane after a global stimulation with a chemoattractant, remain associated with the plasma membrane for an extended period of time in shk1 null cells. These results suggest that SHK1 is a negative regulator of the PI3K (phosphatidylinositol-3 kinase) pathway. Furthermore, when a chemoattractant gradient is applied to a wild-type cell, these PH-domain-containing proteins and the F-actin-binding protein coronin localize to its leading edge, but in an shk1 null cell they become randomly associated with the plasma membrane and cortex, irrespective of the direction of the chemoattractant gradient, suggesting that SHK1 is required for the proper spatiotemporal control of F-actin levels in chemotaxing cells. Consistent with such functions, SHK1 is localized at the plasma membrane/cortex, and we show that its SH2 domain is required for this localization and the proper function of SHK1.
Figures

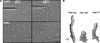
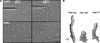
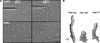





References
-
- Adler K, Gerisch G, von Hugo U, Lupas A, Schweiger A. Classification of tyrosine kinases from Dictyostelium discoideum with two distinct, complete or incomplete catalytic domains. FEBS Lett. 1996;395:286–292. - PubMed
-
- Alessi D, James S, Downes C, Holmes A, Gaffney P, Reese C, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr Biol. 1997;7:261–269. - PubMed
-
- Andjelkovic M, Alessi DR, Meier R, Fernandez A, Lamb NJ, Frech M, Cron P, Cohen P, Lucocq JM, Hemmings BA. Role of translocation in the activation and function of protein kinase B. J Biol Chem. 1997;272:31515–31524. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
