Rapid dephosphorylation of the TorR response regulator by the TorS unorthodox sensor in Escherichia coli
- PMID: 11274133
- PMCID: PMC95190
- DOI: 10.1128/JB.183.8.2691-2695.2001
Rapid dephosphorylation of the TorR response regulator by the TorS unorthodox sensor in Escherichia coli
Abstract
Induction of the torCAD operon, encoding the trimethylamine N-oxide (TMAO) respiratory system, is tightly controlled by the TorS-TorR phosphorelay system in response to TMAO availability. TorS is an unorthodox sensor that contains three phosphorylation sites and transphosphorylates TorR via a four-step phosphorelay, His443-->Asp723-->His850-->Asp(TorR). In this study, we provide genetic evidence that TorS can dephosphorylate phospho-TorR when TMAO is removed. Dephosphorylation probably occurs by a reverse phosphorelay, Asp(TorR)-->His850-->Asp723, since His850 and Asp723 are both essential in this process. By using reverse transcriptase PCR, we also show that TMAO removal results in shutoff of tor operon transcription in less than 2 min. Based on our results and on analogy to other phosphorelay signal transduction systems, we propose that reverse phosphotransfer could be a rapid and efficient mechanism to inactivate response regulators.
Figures
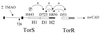



References
-
- Ansaldi M. Régulation multifactorielle du système respiratoire triméthylamine N-oxyde (TMAO) réductase chez Escherichia coli. Ph.D. thesis. Marseille, France: Faculté des Sciences de Luminy, Université Aix-Marseille II; 2000.
-
- Ansaldi M, Bordi C, Lepelletier M, Méjean V. TorC apocytochrome negatively autoregulates the trimethylamine N-oxide (TMAO) reductase operon in Escherichia coli. Mol Microbiol. 1999;33:284–295. - PubMed
-
- Appleby J L, Parkinson J S, Bourret R B. Signal transduction via the multi-step phosphorelay: not necessarily a road less traveled. Cell. 1996;86:845–848. - PubMed
-
- Castelli M E, Garcia V E, Soncini F C. The phosphatase activity is the target for Mg2+ regulation of the sensor protein PhoQ in Salmonella. J Biol Chem. 2000;275:22948–22954. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

