Ca2+-binding activity of a COOH-terminal fragment of the Drosophila BK channel involved in Ca2+-dependent activation
- PMID: 11274367
- PMCID: PMC31910
- DOI: 10.1073/pnas.081072398
Ca2+-binding activity of a COOH-terminal fragment of the Drosophila BK channel involved in Ca2+-dependent activation
Abstract
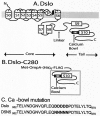
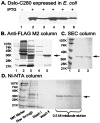
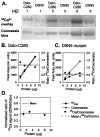
Mutational and biophysical analysis suggests that an intracellular COOH-terminal domain of the large conductance Ca(2+)-activated K(+) channel (BK channel) contains Ca(2+)-binding site(s) that are allosterically coupled to channel opening. However the structural basis of Ca(2+) binding to BK channels is unknown. To pursue this question, we overexpressed the COOH-terminal 280 residues of the Drosophila slowpoke BK channel (Dslo-C280) as a FLAG- and His(6)-tagged protein in Escherichia coli. We purified Dslo-C280 in soluble form and used a (45)Ca(2+)-overlay protein blot assay to detect Ca(2+) binding. Dslo-C280 exhibits specific binding of (45)Ca(2+) in comparison with various control proteins and known EF-hand Ca(2+)-binding proteins. A mutation (D5N5) of Dslo-C280, in which five consecutive Asp residues of the "Ca-bowl" motif are changed to Asn, reduces (45)Ca(2+)-binding activity by 56%. By electrophysiological assay, the corresponding D5N5 mutant of the Drosophila BK channel expressed in HEK293 cells exhibits lower Ca(2+) sensitivity for activation and a shift of approximately +80 mV in the midpoint voltage for activation. This effect is associated with a decrease in the Hill coefficient (N) for activation by Ca(2+) and a reduction in apparent Ca(2+) affinity, suggesting the loss of one Ca(2+)-binding site per monomer. These results demonstrate a functional correlation between Ca(2+) binding to a specific region of the BK protein and Ca(2+)-dependent activation, thus providing a biochemical approach to study this process.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

