Mammalian mad2 and bub1/bubR1 recognize distinct spindle-attachment and kinetochore-tension checkpoints
- PMID: 11274370
- PMCID: PMC31862
- DOI: 10.1073/pnas.081076898
Mammalian mad2 and bub1/bubR1 recognize distinct spindle-attachment and kinetochore-tension checkpoints
Abstract
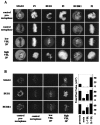
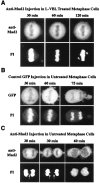
Metaphase checkpoint controls sense abnormalities of chromosome alignment during mitosis and prevent progression to anaphase until proper alignment has been attained. A number of proteins, including mad2, bub1, and bubR1, have been implicated in the metaphase checkpoint control in mammalian cells. Metaphase checkpoints have been shown, in various systems, to read loss of either spindle tension or microtubule attachment at the kinetochore. Characteristically, HeLa cells arrest in metaphase in response to low levels of microtubule inhibitors that leave an intact spindle and a metaphase plate. Here we show that the arrest induced by nanomolar vinblastine correlates with loss of tension at the kinetochore, and that in response the checkpoint proteins bub1 and bubR1 are recruited to the kinetochore but mad2 is not. mad2 remains competent to respond and is recruited at higher drug doses that disrupt spindle association with the kinetochores. Further, although mad2 forms a complex with cdc20, it does not associate with bub1 or bubR1. We conclude that mammalian bub1/bubR1 and mad2 operate as elements of distinct pathways sensing tension and attachment, respectively.
Figures


References
-
- Li R, Murray A W. Cell. 1991;66:519–531. - PubMed
-
- Hoyt M A, Totis L, Roberts B T. Cell. 1991;66:507–517. - PubMed
-
- Amon A. Curr Opin Genet Dev. 1999;9:69–75. - PubMed
-
- Li Y, Benezra R. Science. 1996;274:246–248. - PubMed
-
- Chen R H, Waters J C, Salmon E D, Murray A W. Science. 1996;274:242–246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

