Local osmotic gradients drive the water flux associated with Na(+)/glucose cotransport
- PMID: 11274397
- PMCID: PMC31132
- DOI: 10.1073/pnas.071245198
Local osmotic gradients drive the water flux associated with Na(+)/glucose cotransport
Abstract
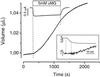
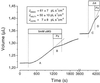
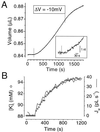
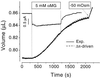
It recently was proposed [Loo, D. D. F., Zeuthen, T., Chandy, G. & Wright, E. M. (1996) Proc. Natl. Acad. Sci. USA 93, 13367--13370] that SGLT1, the high affinity intestinal and renal sodium/glucose cotransporter carries water molecules along with the cosubstrates with a strict stoichiometry of two Na(+), one glucose, and approximately 220 water molecules per transport cycle. Using electrophysiology together with sensitive volumetric measurements, we investigated the nature of the driving force behind the cotransporter-mediated water flux. The osmotic water permeability of oocytes expressing human SGLT1 (L(p) +/- SE) averaged 3.8 +/- 0.3 x 10(-4) cm x s(-1) (n = 15) and addition of 100 microM phlorizin (a specific SGLT1 inhibitor) reduced the permeability to 2.2 +/- 0.2 x 10(-4) cm x s(-1) (n = 15), confirming the presence of a significant water permeability closely associated with the cotransporter. Addition of 5 mM alpha-methyl-glucose (alpha MG) induced an average inward current of 800 +/- 10 nA at -50 mV and a water influx reaching 120 +/- 20 pL cm(-2) x s(-1) within 5-8 min. After rapidly inhibiting the Na(+)/glucose cotransport with phlorizin, the water flux remained significantly elevated, clearly indicating the presence of a local osmotic gradient (Delta pi) estimated at 16 +/- 2 mOsm. In short-term experiments, a rapid depolarization from -100 to 0 mV in the presence of alpha MG decreased the cotransport current by 94% but failed to produce a comparable reduction in the swelling rate. A mathematical model depicting the intracellular accumulation of transported osmolytes can accurately account for these observations. It is concluded that, in SGLT1-expressing oocytes, alpha MG-dependent water influx is induced by a local osmotic gradient by using both endogenous and SGLT1-dependent water permeability.
Figures

Comment in
-
Epithelial water absorption: osmosis or cotransport?Proc Natl Acad Sci U S A. 2001 Mar 27;98(7):3628-30. doi: 10.1073/pnas.081073298. Proc Natl Acad Sci U S A. 2001. PMID: 11274376 Free PMC article. No abstract available.
References
-
- Finkelstein A. Water Movements Through Lipid Bilayers, Pores, and Plasma Membranes: Theory and Reality. New York: Wiley; 1987.
-
- Agre P, Preston G M, Smith B L, Jung J S, Raina S, Moon C, Guggino W B, Nielsen S. Am J Physiol. 1993;265:F463–F476. - PubMed
-
- Loike J D, Hickman S, Kuang K, Xu M, Cao L, Vera J C, Silverstein S C, Fischbarg J. Am J Physiol. 1996;271:C1774–C1779. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

