A splicing switch and gain-of-function mutation in FgfR2-IIIc hemizygotes causes Apert/Pfeiffer-syndrome-like phenotypes
- PMID: 11274405
- PMCID: PMC31142
- DOI: 10.1073/pnas.071586898
A splicing switch and gain-of-function mutation in FgfR2-IIIc hemizygotes causes Apert/Pfeiffer-syndrome-like phenotypes
Abstract
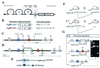
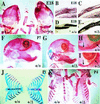
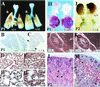
Intercellular signaling by fibroblast growth factors plays vital roles during embryogenesis. Mice deficient for fibroblast growth factor receptors (FgfRs) show abnormalities in early gastrulation and implantation, disruptions in epithelial-mesenchymal interactions, as well as profound defects in membranous and endochondrial bone formation. Activating FGFR mutations are the underlying cause of several craniosynostoses and dwarfism syndromes in humans. Here we show that a heterozygotic abrogation of FgfR2-exon 9 (IIIc) in mice causes a splicing switch, resulting in a gain-of-function mutation. The consequences are neonatal growth retardation and death, coronal synostosis, ocular proptosis, precocious sternal fusion, and abnormalities in secondary branching in several organs that undergo branching morphogenesis. This phenotype has strong parallels to some Apert's and Pfeiffer's syndrome patients.
Figures
Comment in
-
Uncoupling fibroblast growth factor receptor 2 ligand binding specificity leads to Apert syndrome-like phenotypes.Proc Natl Acad Sci U S A. 2001 Mar 27;98(7):3641-3. doi: 10.1073/pnas.081082498. Proc Natl Acad Sci U S A. 2001. PMID: 11274381 Free PMC article. No abstract available.
References
-
- McKeehan W L, Wang F, Kan M. Prog Nucleic Acid Res Mol Biol. 1998;59:135–176. - PubMed
-
- Ornitz D M. BioEssays. 2000;22:108–112. - PubMed
-
- Nishimura T, Nakatake Y, Konishi M, Itoh N. Biochem Biophys Acta. 2000;1492:203–206. - PubMed
-
- Yamashita T, Yoshioka M, Itoh N. Biochem Biophys Res Commun. 2000;277:494–498. - PubMed
-
- Ornitz D M, Xu J S, Colvin J S, McEwen D G, MacArthur C A, Coulier F, Gao G X, Goldfarb M. J Biol Chem. 1996;271:15292–15297. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

