Local gene transfer of tissue factor pathway inhibitor regulates intimal hyperplasia in atherosclerotic arteries
- PMID: 11274432
- PMCID: PMC31182
- DOI: 10.1073/pnas.061004098
Local gene transfer of tissue factor pathway inhibitor regulates intimal hyperplasia in atherosclerotic arteries
Abstract
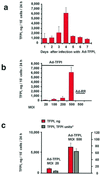
Tissue factor (TF), the initiator of blood coagulation and thrombosis, is up-regulated after vascular injury and in atherosclerotic states. Systemic administration of recombinant TF pathway inhibitor (TFPI) has been reported to decrease intimal hyperplasia after vascular injury and also to suppress systemic mechanisms of blood coagulation and thrombosis. Here we report that, in heritable hyperlipidemic Watanabe rabbits, adenoviral gene transfer of TFPI to balloon-injured atherosclerotic arteries reduced the extent of intimal hyperplasia by 43% (P < 0.05) compared with a control vector used at identical titer (1 x 10(10) plaque-forming units/ml). Platelet aggregation and coagulation studies performed 7 days after local gene transfer of TFPI failed to show any impairment in systemic hemostasis. At time of sacrifice, 4 weeks after vascular injury, the 10 Ad-TFPI treated carotid arteries were free of thrombi, whereas two control-treated arteries were occluded (P, not significant). These findings suggest that TFPI overexpressed in atherosclerotic arteries can regulate hyperplastic response to injury in the absence of changes in the hemostatic system, establishing a role for local TF regulation as target for gene transfer-based antirestenosis therapies.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

