Modular organization of the Friend murine leukemia virus envelope protein underlies the mechanism of infection
- PMID: 11274436
- PMCID: PMC31188
- DOI: 10.1073/pnas.071432398
Modular organization of the Friend murine leukemia virus envelope protein underlies the mechanism of infection
Abstract
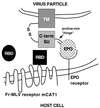
Retrovirus infection is initiated by receptor-dependent fusion of the envelope to the cell membrane. The modular organization of the envelope protein of C type retroviruses has been exploited to investigate how binding of the surface subunit (SU) to receptor triggers fusion mediated by the transmembrane (TM) subunit. We show that deletion of the receptor-binding domain (RBD) from SU of Friend murine leukemia virus (Fr-MLV) abolishes infection that is restored by supplying RBD as a soluble protein. Infection by this mechanism remains dependent on receptor expression. When membrane attachment of the virus lacking RBD is reestablished by inserting the hormone erythropoietin, infection remains dependent on the RBD/receptor complex. However, infection increases 50-fold to 5 x 10(5) units/ml on cells that also express the erythropoietin receptor. Soluble RBD from Fr-MLV also restores infection by amphotropic and xenotropic MLVs in which RBD is deleted. These experiments demonstrate that RBD has two functions: mediating virus attachment and activating the fusion mechanism. In addition, they indicate that receptor engagement triggers fusion by promoting a subgroup-independent functional interaction between RBD and the remainder of SU and/or TM.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

