Differences in quantal amplitude reflect GluR4- subunit number at corticothalamic synapses on two populations of thalamic neurons
- PMID: 11274440
- PMCID: PMC31198
- DOI: 10.1073/pnas.061013698
Differences in quantal amplitude reflect GluR4- subunit number at corticothalamic synapses on two populations of thalamic neurons
Abstract
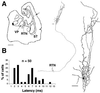
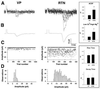
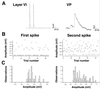
Low-frequency thalamocortical oscillations that underlie drowsiness and slow-wave sleep depend on rhythmic inhibition of relay cells by neurons in the reticular nucleus (RTN) under the influence of corticothalamic fibers that branch to innervate RTN neurons and relay neurons. To generate oscillations, input to RTN predictably should be stronger so disynaptic inhibition of relay cells overcomes direct corticothalamic excitation. Amplitudes of excitatory postsynaptic conductances (EPSCs) evoked in RTN neurons by minimal stimulation of corticothalamic fibers were 2.4 times larger than in relay neurons, and quantal size of RTN EPSCs was 2.6 times greater. GluR4-receptor subunits labeled at corticothalamic synapses on RTN neurons outnumbered those on relay cells by 3.7 times, providing a basis for differences in synaptic strength.
Figures



Comment in
-
The GABAergic reticular nucleus: a preferential target of corticothalamic projections.Proc Natl Acad Sci U S A. 2001 Mar 27;98(7):3625-7. doi: 10.1073/pnas.071051998. Epub 2001 Mar 20. Proc Natl Acad Sci U S A. 2001. PMID: 11259667 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

